Controlled Synthesis of Monodisperse Hexagonal NaYF4:Yb/Er Nanocrystals with Ultrasmall Size and Enhanced Upconversion Luminescence
Abstract
:1. Introduction
2. Results and Discussion
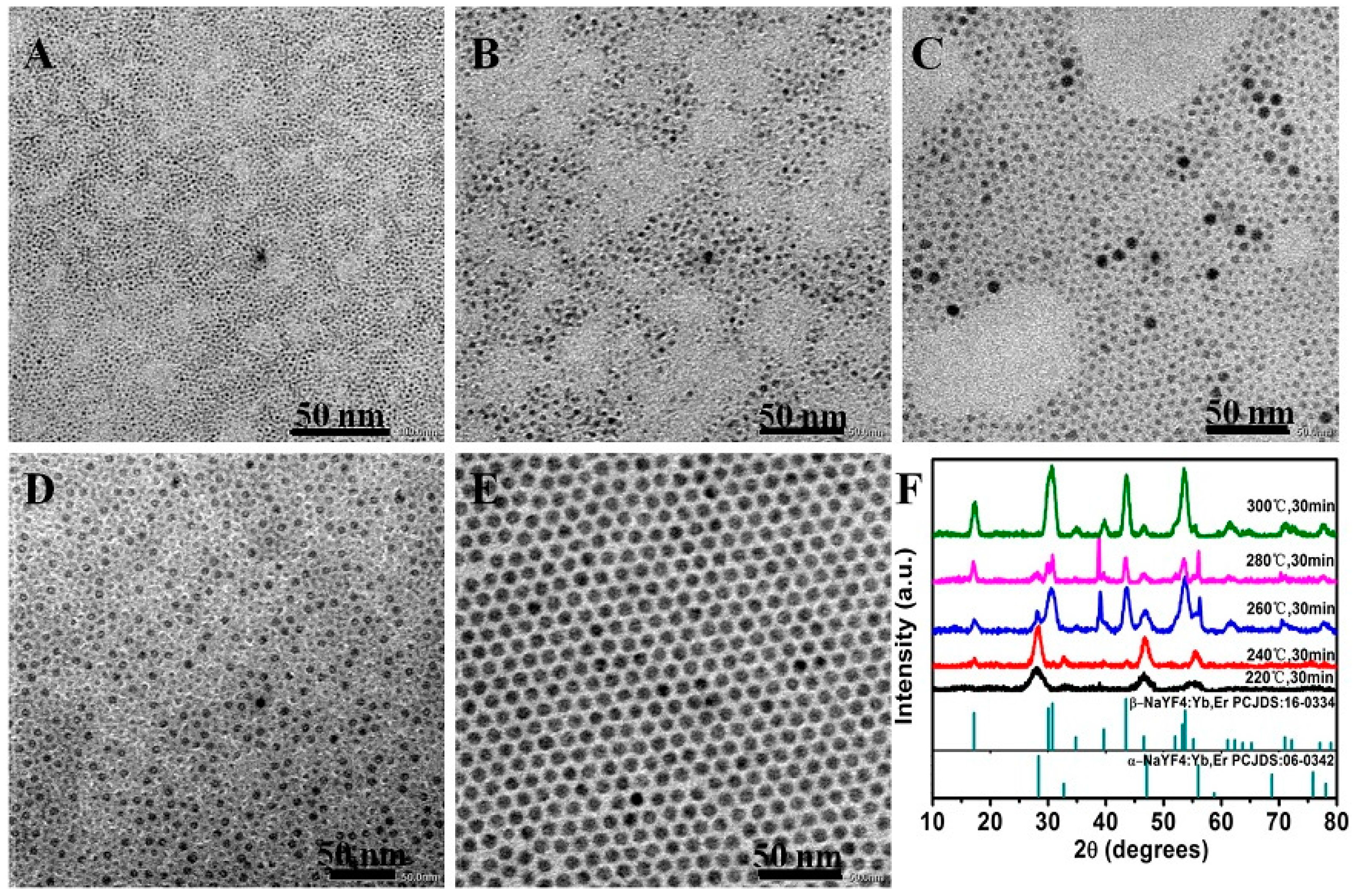
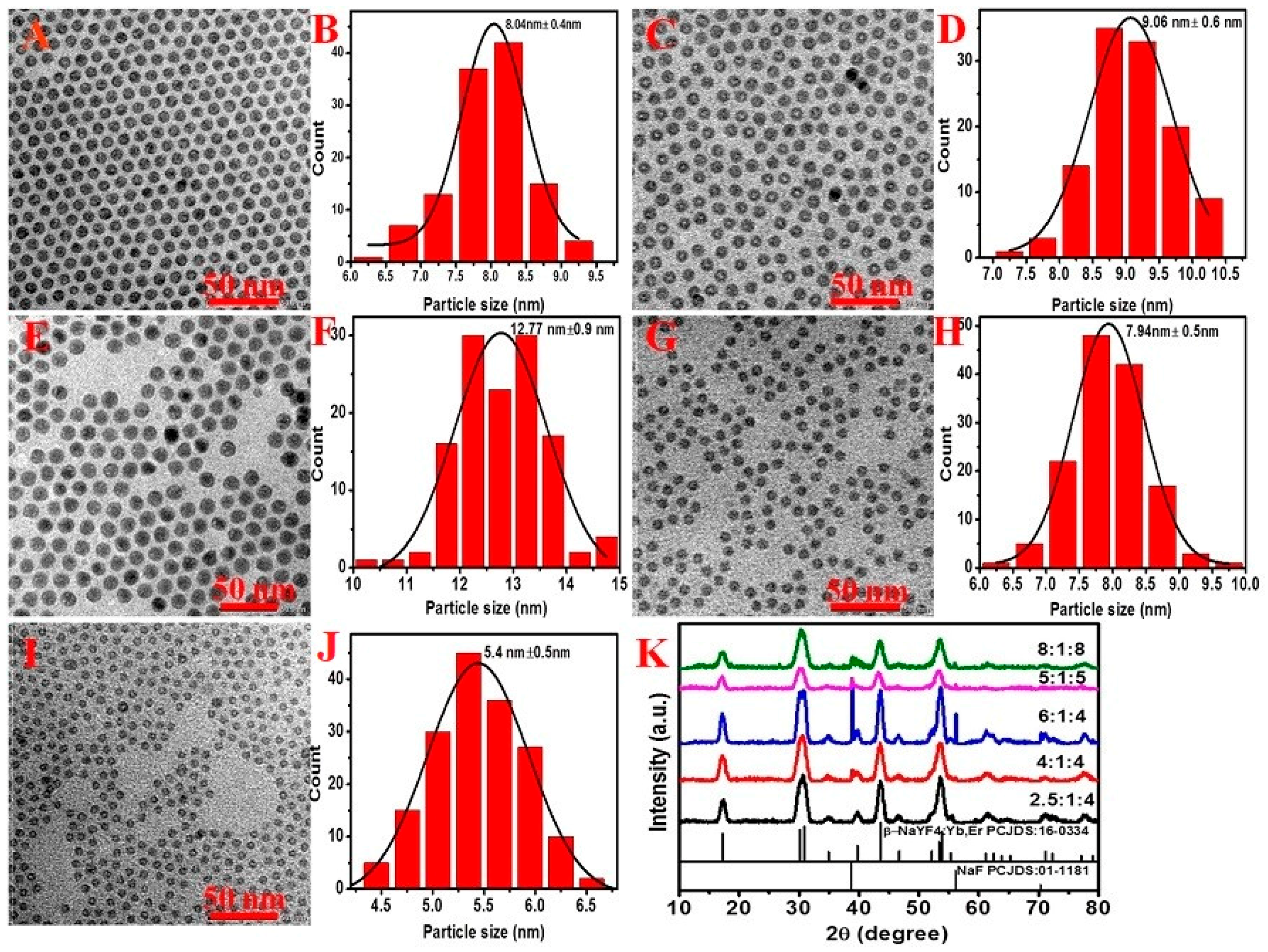
2.1. Control of the Phase and Size of NaYF4:Yb/Er Nanoparticles
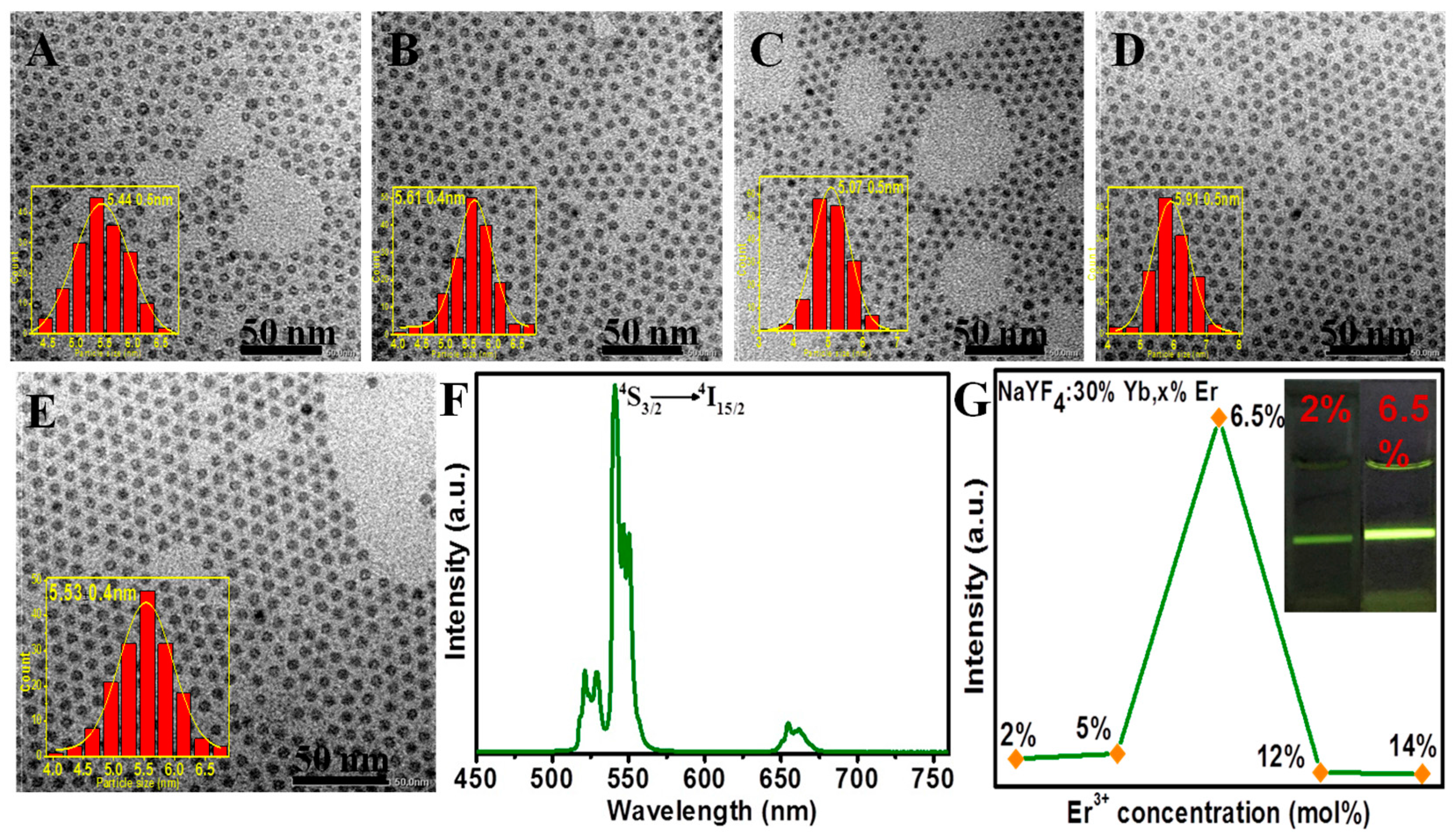
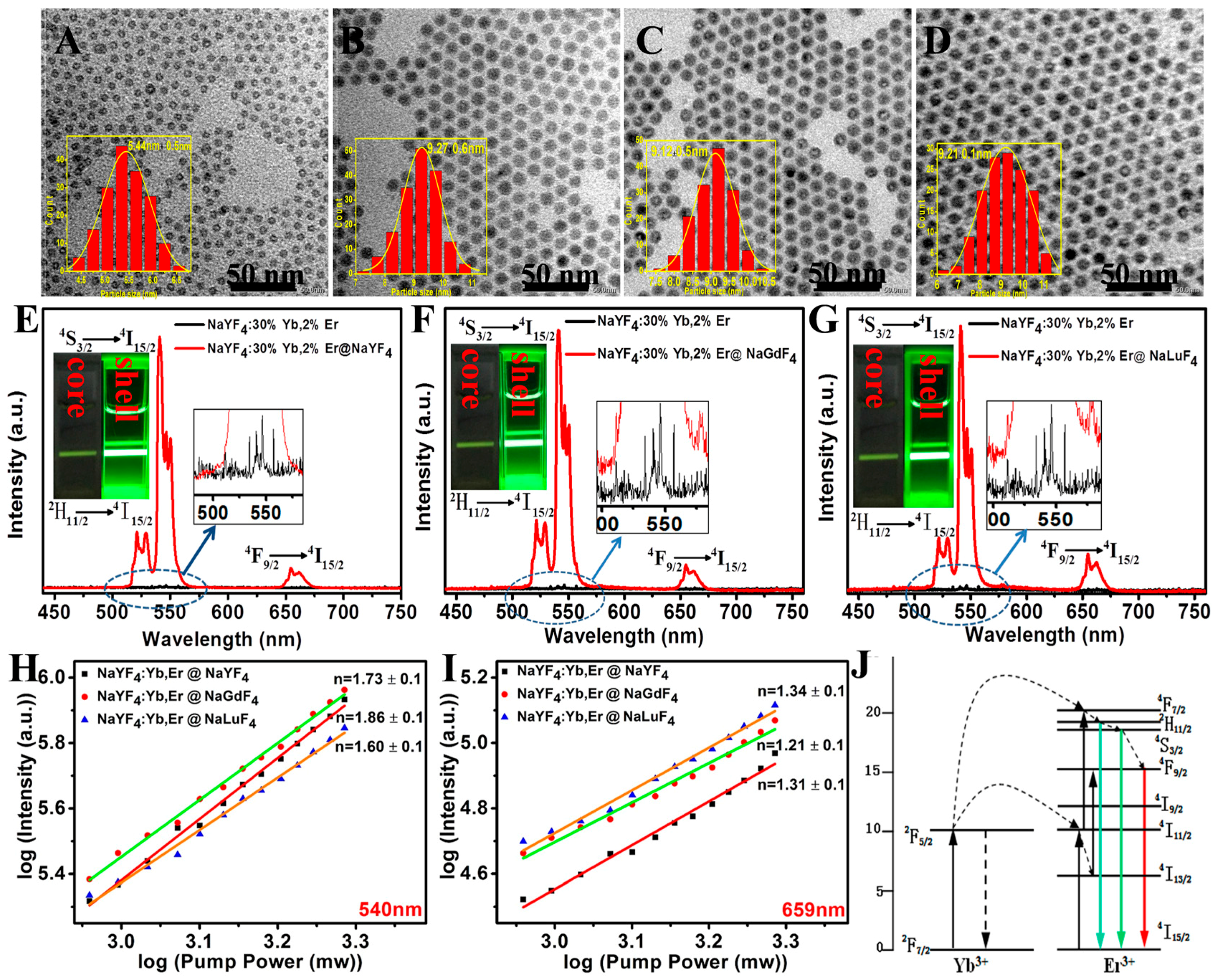
2.2. Enhancing Upconversion Luminescence of Ultrasmall β-NaYF4:Yb/Er
3. Materials and Methods
3.1. Materials
3.2. Synthesize Rare-Earth Oleates
3.3. Synthesis of β-NaYF4:Yb3+/Er3+ UCNCs
3.4. Synthesis of NaYF4:Yb3+/Er3+@NaREF4 UCNCs
3.5. Instrumentation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, G.Y.; Agren, H.; Ohulchanskyya, T.Y.; Prasad, P.N. Light upconverting core–shell nanostructures: Nanophotonic control for emerging applications. Chem. Soc. Rev. 2015, 44, 1680–1713. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, D.; Lasher, M.; Fainman, Y. Fluorescent volumetric display excited by a single infrared beam. Appl. Opt. 2005, 44, 5281–5285. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Gu, Z.; Zhou, L.; Yin, W.; Liu, X.; Yan, L.; Jin, S.; Ren, W.; Xing, G.; Li, S.; et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Heer, S.; Kompe, K.; Gudel, H.U.; Haase, M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Yang, T.; Feng, W.; Li, C.; Li, F. Sub-10 nm hexagonal lanthanide-doped NaLuF4 upconversion nanocrystals for sensitive bioimaging in Vivo. J. Am. Chem. Soc. 2011, 133, 17122–17125. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X.G. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide doped NaYF4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 5642–5643. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Yan, R.X.; Hao, Z.Y.; Wang, L.; Zeng, J.H.; Bao, J.; Wang, X.; Peng, Q.; Li, Y.D. Fluorescence resonant energy transfer biosensor based on upconversion-luminescent nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 6054–6057. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.M.; Zhou, J.; Liu, J.L.; Feng, W.; Li, F.Y. Iridium-complex-modified upconversion nanophosphors for effective LRET detection of cyanide anions in pure water. Adv. Funct. Mater. 2012, 22, 2667–2672. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.H.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of nanoparticles. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.Y.; Yang, Y.; Sun, Y.; Wu, Y.Q.; Gao, Y.; Feng, W.; Li, F.Y. Biodistribution of sub-10nm PEG-modified radioactive/upconversion nanoparticles. Biomaterials 2013, 34, 7127–7134. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Valiev, R.R.; Ohulchanskyy, T.Y.; Ågren, H.; Yang, C.H.; Chen, G.Y. Dye-Sensitized Lanthanide-Doped Upconversion Nanoparticles. Chem. Soc. Rev. 2017, 46, 4150–4167. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Zhang, Y.; Jiang, S. Multi-color core–shell structured upconversion fluorescent nanoparticles. Adv. Mater. 2008, 20, 4765–4769. [Google Scholar] [CrossRef]
- Boyer, J.C.; Carling, C.J.; Gates, B.D.; Branda, N.R. Two-way photoswitching using one type of near-infrared light, upconverting nanoparticles, and changing only the light intensity. J. Am. Chem. Soc. 2010, 132, 15766–15772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.X.; Liu, B.C.; Chai, Z.L.; Xu, G.R.; Yu, S.L.; Zhang, J. Synthesis of NaYF4:Eu3+/Tb3+ nanostructures with diverse morphologies and their size- and morphology-dependent photoluminescence. CrystEngComm 2013, 15, 8262–8272. [Google Scholar] [CrossRef]
- Mai, H.X.; Zhang, Y.W.; Si, R.; Yan, Z.G.; Sun, L.G.; You, L.P.; Yan, C.H. Highly-quality sodium rare-earth fluoride nanocrystals: Controlled synthesis and optical properties. J. Am. Chem. Soc. 2006, 128, 6426–6436. [Google Scholar] [CrossRef] [PubMed]
- Voss, B.; Haase, M. Intrinsic focusing of the particle size distribution in colloids containing nanocrystals of two different crystal phases. ACS Nano 2013, 7, 11242–11254. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, G.Y.; Hao, S.W.; Yang, C.H. Sub-6 nm monodisperse hexagonal core/shell NaGdF4 nanocrystals with enhanced upconversion photoluminescence. Nanoscale 2017, 9, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.H.; Wang, J.; Xu, J.; Chen, H.Y.; Zhang, C.; Hong, M.H.; Liu, X.G. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhao, Y. Sub-10 nm and monodisperse β-NaYF4:Yb,Tm,Gd nanocrystals with intense ultraviolet upconversion luminescence. J. Mater. Chem. C 2014, 2, 2198–2203. [Google Scholar] [CrossRef]
- Podhorodecki, A. Designing, synthesis and optical properties of ultrasmall β-NaYF4 nanocrystals doped by lanthanides ions for bio-medical applications. ECS Trans. 2012, 45, 191–198. [Google Scholar] [CrossRef]
- Ostrowski, A.D.; Chan, E.M.; Gargas, D.J.; Katz, E.M.; Han, G.; Schuck, P.J.; Milliron, D.J.; Cohen, B.E. Controlled synthesis and single-particle imaging of bright, sub-10 nm lanthanide-doped upconverting nanocrystals. ACS Nano 2012, 6, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Rinkel, T.; Raj, A.N.; Duhnen, S.; Haase, M. Synthesis of 10 nm β-NaYF4:Yb,Er/NaYF4 core/shell upconversion nanocrystals with 5 nm particle cores. Angew. Chem. Int. Ed. 2016, 55, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.S.; Chow, G.M. Synthesis of hexagonal-phase NaYF4:Yb,Er and NaYF4:Yb,Tm nanocrystals with efficient up-conversion fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Liu, X. Direct evidence of a surface quenching effect on size-dependent luminescence of upconversion nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7456–7460. [Google Scholar] [CrossRef] [PubMed]
- Dühnen, S.; Rinkel, T.; Haase, M. Size control of nearly monodisperse β-NaGdF4 particles prepared from small α-NaGdF4 nanocrystals. Chem. Mater. 2015, 27, 4033–4039. [Google Scholar] [CrossRef]
- Rinkel, T.; Nordmann, J.; Raj, A.N.; Haase, M. Ostwald-ripening and particle size focussing of sub-10 nm NaYF4 upconversion nanocrystals. Nanoscale 2014, 6, 14523–14530. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Ohulchanskyy, T.Y.; Kumar, R.; Agren, H.; Prasad, P.N. Ultrasmall monodisperse NaYF4:Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hao, S.W.; Yang, C.H.; Chen, G.Y. Synthesis of multicolor core/shell NaLuF4:Yb3+/Ln3+@CaF2 upconversion nanocrystals. Nanomaterials 2017, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.C.; Vetrone, F.; Cuccia, L.A.; Capobianco, J.A. Synthesis of colloidal upconverting NaYF4 nanocrystals doped with Er3+,Yb3+ and Tm3+,Yb3+ via thermal decomposition of lanthanide trifluoroacetate precursors. J. Am. Chem. Soc. 2006, 128, 7444–7445. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xu, L.; Chen, G. Controlled Synthesis of Monodisperse Hexagonal NaYF4:Yb/Er Nanocrystals with Ultrasmall Size and Enhanced Upconversion Luminescence. Molecules 2017, 22, 2113. https://doi.org/10.3390/molecules22122113
Li H, Xu L, Chen G. Controlled Synthesis of Monodisperse Hexagonal NaYF4:Yb/Er Nanocrystals with Ultrasmall Size and Enhanced Upconversion Luminescence. Molecules. 2017; 22(12):2113. https://doi.org/10.3390/molecules22122113
Chicago/Turabian StyleLi, Hui, Lei Xu, and Guanying Chen. 2017. "Controlled Synthesis of Monodisperse Hexagonal NaYF4:Yb/Er Nanocrystals with Ultrasmall Size and Enhanced Upconversion Luminescence" Molecules 22, no. 12: 2113. https://doi.org/10.3390/molecules22122113




