Theoretical Kinetic and Mechanistic Studies on the Reactions of CF3CBrCH2 (2-BTP) with OH and H Radicals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Abstraction and Substitution
2.1.1. CF3CBrCH2 + OH
2.1.2. CF3CBrCH2 + H
2.2. Addition Mechanism
2.2.1. CF3CBrCH2 + OH
2.2.2. CF3CBrCH2 + H
2.3. Kinetic Calculations
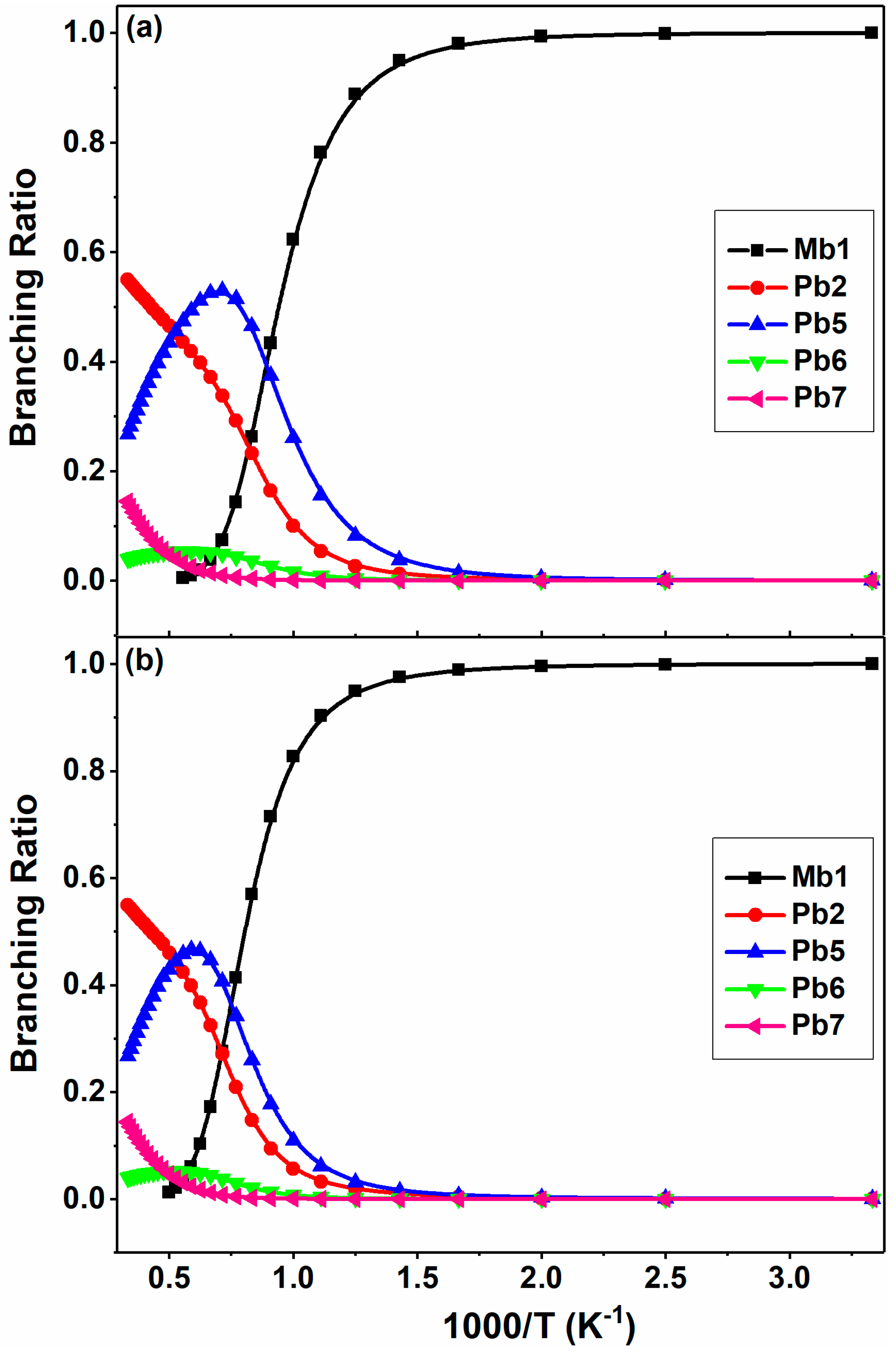
2.3.1. CF3CBrCH2 + OH
2.3.2. CF3CBrCH2 + H
3. Theoretical Methodology
4. Conclusions
Supplementary Materials
| Order | Filename | Content |
|---|---|---|
| 1 | Supplemental Material-I |
|
| 2 | Supplemental Material-II | Cartesian coordinates and frequencies of all the species involved in the present work. |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burkholder, J.B.; Cox, R.A.; Ravishankara, A.R. Atmospheric Degradation of Ozone Depleting Substances, Their Substitutes, and Related Species. Chem. Rev. 2015, 115, 3704–3759. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.J.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atomc-atalysed destruction of ozone. Nature 1974, 249, 810–812. [Google Scholar] [CrossRef]
- Pagliaro, J.L.; Linteris, G.T.; Babushok, V.I. Premixed flame inhibition by C2HF3Cl2 and C2HF5. Combust. Flame 2016, 163, 54–65. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, X.; Han, Y. Theoretical and experimental studies on the atmospheric degradation of 2-bromo-3,3,3-trifluoropropene. Phys. Chem. Chem. Phys. 2015, 17, 20543–20550. [Google Scholar] [CrossRef] [PubMed]
- Meiling, Z.; Ce, S.; Yan, T. Comprehensive Theoretical Studies on the Reaction of 1-Bromo-3,3,3-trifluoropropene with OH Free Radicals. Molecules 2013, 18, 7873–7885. [Google Scholar] [CrossRef]
- Orkin, V.L.; Louis, F.; Huie, R.E.; Kurylo, M.J. Photochemistry of Bromine-Containing Fluorinated Alkenes: Reactivity toward OH and UV Spectra. J. Phys. Chem. A 2002, 106, 10195–10199. [Google Scholar] [CrossRef]
- Linteris, G.T.; Babushok, V.I.; Pagliaro, J.L.; Burgess, J.D.R.; Manion, J.A.; Takahashi, F.; Katta, V.R.; Baker, P.T. Understanding overpressure in the FAA aerosol can test by C3H2F3Br (2-BTP). Combust. Flame 2016, 167, 452–462. [Google Scholar] [CrossRef]
- Takahashi, F.; Katta, V.R.; Linteris, G.T.; Babushok, V.I. Combustion inhibition and enhancement of cup-burner flames by CF3Br, C2HF5, C2HF3Cl2, and C3H2F3Br. Proc. Combust. Inst. 2015, 35, 2741–2748. [Google Scholar] [CrossRef]
- Pagliaro, J.L.; Bouvet, N.; Linteris, G.T. Premixed flame inhibition by CF3Br and C3H2F3Br (2-BTP). Combust. Flame 2016, 169, 272–286. [Google Scholar] [CrossRef]
- Burgess, D.R.; Babushok, V.I.; Linteris, G.T.; Manion, J.A. A Chemical Kinetic Mechanism for 2-Bromo-3,3,3-trifluoropropene (2-BTP) Flame Inhibition. Int. J. Chem. Kinet. 2015, 47, 533–563. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linteris, G.T.; Burgess, D.R., Jr.; Baker, P.T. Hydrocarbon flame inhibition by C3H2F3Br (2-BTP). Combust. Flame 2015, 162, 1104–1112. [Google Scholar] [CrossRef]
- Vahdat, N.; Zou, Y.; Collins, M. Fire-extinguishing effectiveness of new binary agents. Fire Saf. J. 2003, 38, 553–567. [Google Scholar] [CrossRef]
- Ni, X.; Chow, W.; Li, Q.; Tao, C. Experimental study of new gas-solid composite particles in extinguishing cooking oil fires. J. Fire Sci. 2011, 29, 152–176. [Google Scholar]
- Zhou, X.; Chen, W.; Chao, M.; Liao, G. The study of thermal decomposition of 2-bromo-3,3,3-trifluoropropene and its fire-extinguishing mechanism. J. Fluor. Chem. 2013, 153, 101–106. [Google Scholar] [CrossRef]
- Gann, R.G.; Burgess, S.R.; Whisner, K.C.; Reneke, P.A. Papers from 1991–2006 Halon Options Technical Working Conferences (HOTWC) [CD-ROM]; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2006.
- Zou, Y.; Vahdat, N.; Collins, M. Fire Protection with Bromoalkene/Nitrogen Gaseous Mixtures. Ind. Eng. Chem. Res. 2001, 40, 4649–4653. [Google Scholar] [CrossRef]
- Dixon-Lewis, G.; Simpson, R.J. Aspects of flame inhibition by halogen compounds. Symp. Int. Combust. 1977, 16, 1111–1119. [Google Scholar] [CrossRef]
- Reinhardt, J.W.; Administration, U.S.F.A. Behavior of Bromotrifluoropropene and Pentafluoroethane when Subjected to a Simulated Aerosol Can Explosion; U.S. Department of Transportation, Federal Aviation Administration: Washington, DC, USA, 2004.
- Enstice, E.C.; Duncan, J.R.; Setser, D.W.; Holmes, B.E. Unimolecular Reactions in the CF3CH2Cl ↔ CF2ClCH2F System: Isomerization by Interchange of Cl and F Atoms. J. Phys. Chem. A 2011, 115, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, C.E.; Duncan, J.R.; Heard, G.L.; Setser, D.W.; Holmes, B.E. Unimolecular Reactions of Chemically Activated CF2BrCF2CH3 and CF2BrCF2CD3: Evidence for 1,2-FBr Interchange. J. Phys. Chem. A 2008, 112, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.K.; Rossabi, S.M.; McClintock, C.E.; Heard, G.L.; Setser, D.W.; Holmes, B.E. Unimolecular Isomerization of CH2FCD2Cl via the Interchange of Cl and F Atoms: Assignment of the Threshold Energy to the 1,2-Dyotropic Rearrangement. J. Phys. Chem. A 2013, 117, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Patten, K.O.; Khamaganov, V.G.; Orkin, V.L.; Baughcum, S.L.; Wuebbles, D.J. OH reaction rate constant, IR absorption spectrum, ozone depletion potentials and global warming potentials of 2-bromo-3,3,3-trifluoropropene. J. Geophys. Res. Atmos. 2011, 116, D24307. [Google Scholar] [CrossRef]
- Nizovtsev, A.S.; Bogdanchikov, G.A.; Baklanov, A.V. The computational study of the “inversion substitution” reactions CX3Br + O2 → CX3O2 + Br (X = H, F). Combust. Flame 2010, 157, 1382–1389. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Wilson, A.K.; Woon, D.E.; Peterson, K.A.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. IX. The atoms gallium through krypton. J. Chem. Phys. 1999, 110, 7667–7676. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Purvis, G.D., III; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A fifth-order perturbation comparison of electron correlation theories. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Zhang, M.; Hao, Y.; Guo, Y.; Xie, Y.; Schaefer, H.F. Anchoring the potential energy surface for the Br + H2O → HBr + OH reaction. Theor. Chem. Acc. 2014, 133, 1513. [Google Scholar] [CrossRef]
- Lee, E.P.F.; Dyke, J.M.; Chau, F.T.; Chow, W.K.; Mok, D.K.W. Reaction enthalpies and activation energies of two important reactions in flame suppression by CF3Br. Chem. Phys. Lett. 2003, 376, 465–474. [Google Scholar] [CrossRef]
- Moore, C.E. Atomic Energy Levels; US Government Printing Office: Washington, DC, USA, 1959; Volume III.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A. Gaussian. In Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Eckart, C. The Penetration of a Potential Barrier by Electrons. Phys. Rev. 1930, 35, 1303–1309. [Google Scholar] [CrossRef]
- Jiao, Y.; Dibble, T.S. Quality Structures, Vibrational Frequencies, and Thermochemistry of the Products of Reaction of BrHg(•) with NO2, HO2, ClO, BrO, and IO. J. Phys. Chem. A 2015, 119, 10502–10510. [Google Scholar] [CrossRef] [PubMed]
- Saheb, V.; Pourhaghighi, N.Y. Theoretical Kinetics Studies on the Reaction of CF3CF=CF2 with Hydroxyl Radical. J. Phys. Chem. A 2014, 118, 9941–9950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Chao, K.; Sun, H.; Wang, F.; Tang, S.; Pan, X.; Zhang, J.; Wang, R. Mechanistic and Kinetic Study of CF3CH=CH2 + OH Reaction. J. Phys. Chem. A 2012, 116, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Zellner, R.; Benson, S.W. Thermochemical kinetics, Ber. Bunsenges. Phys. Chem. 1977, 81, 877–878. [Google Scholar] [CrossRef]
- Benson, S.W.; Cruickshank, F.R.; Golden, D.M.; Haugen, G.R.; O’Neal, H.E.; Rodgers, A.S.; Shaw, R.; Walsh, R. Additivity rules for the estimation of thermochemical properties. Chem. Rev. 1969, 69, 279–324. [Google Scholar] [CrossRef]
- Hippler, H.; Troe, J.; Wendelken, H. Collisional deactivation of vibrationally highly excited polyatomic molecules. II. Direct observations for excited toluene. J. Chem. Phys. 1983, 78, 6709–6717. [Google Scholar] [CrossRef]
- Ye, L.; Georgievskii, Y.; Klippenstein, S.J. Pressure-dependent branching in the reaction of 1CH2 with C2H4 and other reactions on the C3H6 potential energy surface. Proc. Combust. Inst. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Ye, L.; Xing, L.; Yuan, W.; Li, Y.; Zhang, L.; Qi, F. Predictive kinetics on the formation and decomposition of ethylbenzene. Proc. Combust. Inst. 2017, 36, 533–542. [Google Scholar] [CrossRef]
- Georgievskii, Y.; Miller, J.A.; Burke, M.P.; Klippenstein, S.J. Reformulation and Solution of the Master Equation for Multiple-Well Chemical Reactions. J. Phys. Chem. A 2013, 117, 12146–12154. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |













© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, H.; Ye, L.; Sun, J. Theoretical Kinetic and Mechanistic Studies on the Reactions of CF3CBrCH2 (2-BTP) with OH and H Radicals. Molecules 2017, 22, 2140. https://doi.org/10.3390/molecules22122140
Bian H, Ye L, Sun J. Theoretical Kinetic and Mechanistic Studies on the Reactions of CF3CBrCH2 (2-BTP) with OH and H Radicals. Molecules. 2017; 22(12):2140. https://doi.org/10.3390/molecules22122140
Chicago/Turabian StyleBian, Huiting, Lili Ye, and Jinhua Sun. 2017. "Theoretical Kinetic and Mechanistic Studies on the Reactions of CF3CBrCH2 (2-BTP) with OH and H Radicals" Molecules 22, no. 12: 2140. https://doi.org/10.3390/molecules22122140