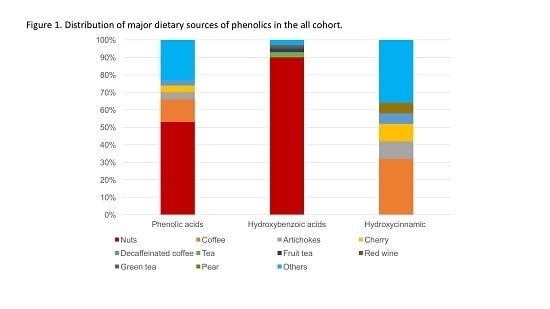
Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Data Collection
4.3. Dietary Assessment
4.4. Estimation of Polyphenol Intake
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dy, G.W.; Gore, J.L.; Forouzanfar, M.H.; Naghavi, M.; Fitzmaurice, C. Global Burden of Urologic Cancers, 1990–2013. Eur. Urol. 2017, 71, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cubero, M.J.; Pascual-Geler, M.; Rivas, A.; Martinez-Gonzalez, L.J.; Saiz, M.; Lorente, J.A.; Cozar, J.M. Lifestyle and dietary factors in relation to prostate cancer risk. Int. J. Food Sci. Nutr. 2015, 66, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Sciacca, S.; Pajak, A.; Martinez-Gonzalez, M.A.; Giovannucci, E.L.; Galvano, F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: A dose-response meta-analysis. Eur. J. Epidemiol. 2016, 31, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.C.; Severi, G.; Baglietto, L.; Krishnan, K.; English, D.R.; Hopper, J.L.; Giles, G.G. Dietary patterns and prostate cancer risk. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Bella, F.; Sciacca, S.; Galvano, F.; Grosso, G. Vegetarianism and breast, colorectal and prostate cancer risk: An overview and meta-analysis of cohort studies. J. Hum. Nutr. Diet. 2017, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Russo, G.I.; De Nunzio, C.; Sebastianelli, A.; Salvi, M.; Vignozzi, L.; Tubaro, A.; Morgia, G.; Serni, S. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. 2017, 20, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Regis, F.; Castelli, T.; Favilla, V.; Privitera, S.; Giardina, R.; Cimino, S.; Morgia, G. A Systematic Review and Meta-analysis of the Diagnostic Accuracy of Prostate Health Index and 4-Kallikrein Panel Score in Predicting Overall and High-grade Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, 429–439.e1. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Rio, D.D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Shen, Y.; Li, X.; Guo, H. The association of tea consumption and the risk and progression of prostate cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 3881–3891. [Google Scholar] [PubMed]
- Liu, H.; Hu, G.H.; Wang, X.C.; Huang, T.B.; Xu, L.; Lai, P.; Guo, Z.F.; Xu, Y.F. Coffee consumption and prostate cancer risk: A meta-analysis of cohort studies. Nutr. Cancer 2015, 67, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Rapisarda, G.; Marventano, S.; Galvano, F.; Mistretta, A.; Grosso, G. Association between polyphenol intake and adherence to the Mediterranean diet in Sicily, southern Italy. NFS J. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Grosso, G.; Yang, J.; Marventano, S.; Micek, A.; Galvano, F.; Kales, S.N. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: A systematic review and meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2015, 101, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Estruch, R. Nut consumption and age-related disease. Maturitas 2016, 84, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Marventano, S.; Antoci, M.; Cagnetti, A.; Castorina, G.; Galvano, F.; Marranzano, M.; Mistretta, A. Coffee and metabolic impairment: An updated review of epidemiological studies. NFS J. 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Eroglu, C.; Secme, M.; Bagci, G.; Dodurga, Y. Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumour Biol. 2015, 36, 9437–9446. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Singh, S.; Burke, T.R., Jr.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.P.; Lin, C.Y.; Huo, C.; Hsiao, P.H.; Su, L.C.; Jiang, S.S.; Chan, T.M.; Chang, C.H.; Chen, L.T.; Kung, H.J.; et al. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1. Oncotarget 2015, 6, 6684–6707. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Salomone, F.; Godos, J.; Pluchinotta, F.; Del Rio, D.; Mistretta, A.; Grosso, G. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2016, 35, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur. J. Nutr. 2017, 56, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Di Mauro, M.; Regis, F.; Reale, G.; Campisi, D.; Marranzano, M.; Lo Giudice, A.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Association between dietary phytoestrogens intakes and prostate cancer risk in Sicily. Aging Male 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Marventano, S.; D’Urso, M.; Mistretta, A.; Galvano, F. The Mediterranean healthy eating, ageing, and lifestyle (MEAL) study: Rationale and study design. Int. J. Food Sci. Nutr. 2017, 68, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Mistretta, A.; Platania, A.; Galvano, F.; Grosso, G. Reliability and relative validity of a food frequency questionnaire for Italian adults living in Sicily, Southern Italy. Int. J. Food Sci. Nutr. 2016, 67, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a food frequency questionnaire for use in Italian adults living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Marventano, S.; Mistretta, A.; Galvano, F.; Grosso, G. Dietary sources of polyphenols in the Mediterranean healthy Eating, Aging and Lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2017, 68, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
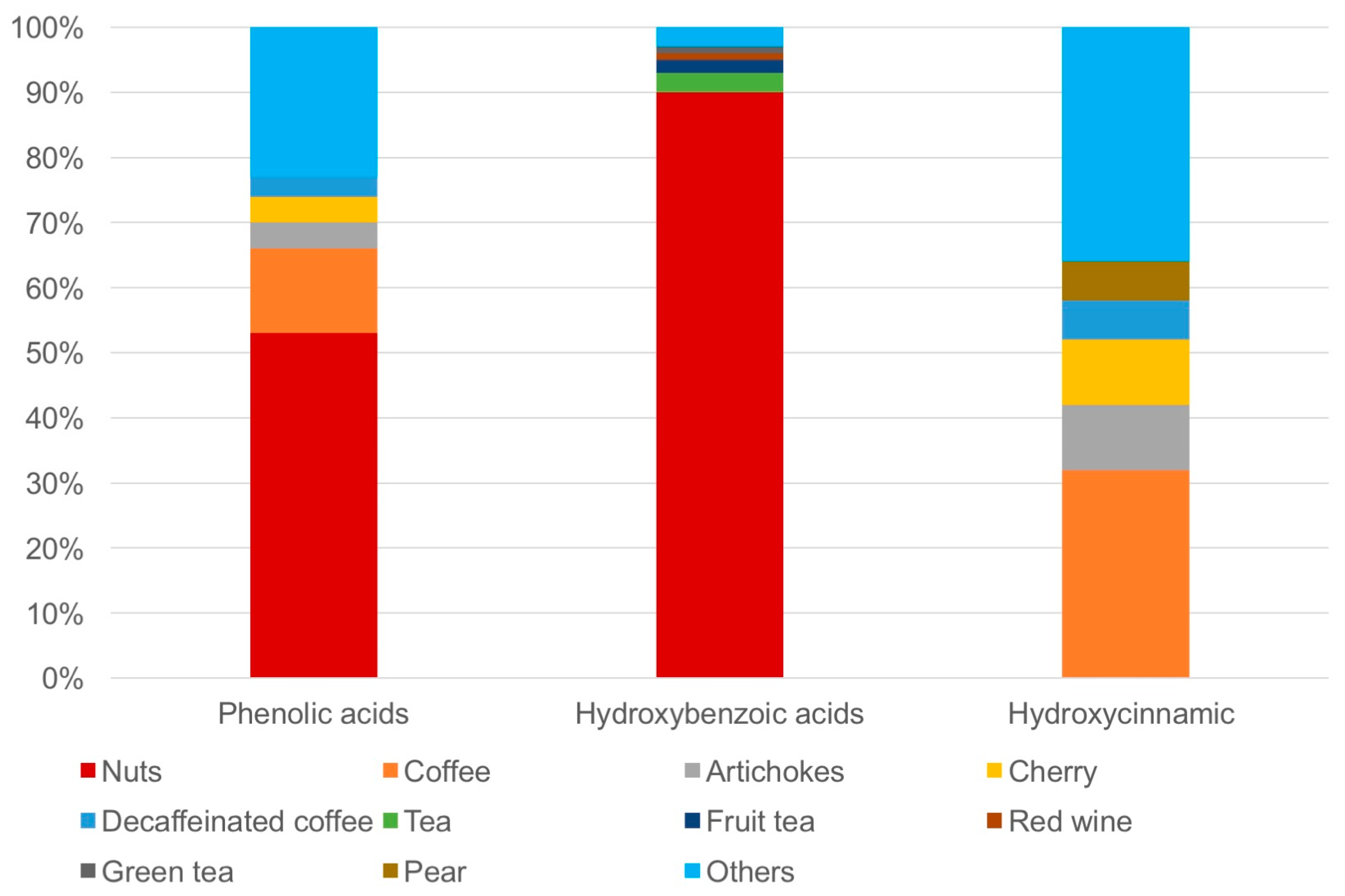
| Cases (n = 118) | Controls (n = 222) | p-Value | |
|---|---|---|---|
| Age (y), mean (SD) | 69.13 (6.60) | 68.09 (8.18) | 0.19 |
| BMI, mean (SD) | 26.49 (3.34) | 27.49 (3.28) | 0.30 |
| Weight status, n (%) | |||
| Normal | 42 (35.6%) | 59 (26.6%) | |
| Overweight | 60 (50.8%) | 127 (57.2%) | |
| Obese | 16 (13.6%) | 36 (16.2%) | |
| Smoking status, n (%) | 0.15 | ||
| Non-smoker | 68 (57.6%) | 143 (64.4%) | |
| Current smoker | 50 (42.4%) | 79 (35.6%) | |
| Alcohol intake, n (%) | 0.16 | ||
| <12 g/day | 55 (46.6%) | 153 (68.9%) | |
| ≥12 g/day | 63 (53.4%) | 69 (31.1%) | |
| Education, n (%) | 0.11 | ||
| Primary/secondary | 96 (81.4%) | 49 (22.1%) | |
| High school/university | 22 (18.6%) | 173 (77.9%) | |
| Physical activity level, n (%) | 0.21 | ||
| Low | 38 (32.2%) | 49 (26.2%) | |
| Medium | 64 (54.2%) | 67 (35.8%) | |
| High | 16 (13.6%) | 71 (38.0%) | |
| Family history of prostatic cancer, n (%) | 43 (36.44%) | 9 (4.05%) | <0.01 |
| Cases (n = 118), Mean SD | Controls (n = 222), Mean SD | p-Value | |
|---|---|---|---|
| Total phenolic acids | 383.41 (522.77) | 400.20 (540.07) | 0.78 |
| Subclasses | |||
| Hydroxybenzoic acids | 218.46 (487.25) | 238.29 (525.82) | 0.73 |
| Hydroxycinammic acid | 164.04 (106.69) | 160.95 (97.57) | 0.78 |
| Hydroxyphenilacetic acid | 0.64 (0.56) | 0.65 (1.07) | 0.34 |
| Individual phenolic acids | |||
| Caffeic acid | 2.76 (1.84) | 2.28 (2.40) | 0.05 |
| Cinnamic acid | 0.48 (0.60) | 0.57 (1.81) | 0.60 |
| Vanillic acid | 0.52 (0.38) | 0.46 (0.59) | 0.34 |
| Ferulic acid | 4.04 (3.35) | 2.80 (2.57) | <0.001 |
| Phenolic Acid Quartiles, OR (95% CI) | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Total phenolic acids | ||||
| No. of cases | 26 | 35 | 26 | 31 |
| OR (95% CI) a | Ref. | 1.30 (0.68–2.47) | 0.87 (0.44–1.69) | 0.81 (0.41–1.62) |
| OR (95% CI) b | Ref. | 1.21 (0.48–3.00) | 0.65 (0.24–1.69) | 1.02 (0.37–2.78) |
| Hydroxybenzoic acids | ||||
| No. of cases | 31 | 21 | 32 | 34 |
| OR (95% CI) a | Ref. | 0.70 (0.35–1.37) | 0.99 (0.53–1.86) | 0.88 (0.47–1.67) |
| OR (95% CI) b | Ref. | 0.77 (0.30–2.01) | 0.46 (0.18–1.18) | 0.75 (0.29–1.94) |
| Hydroxycinammic acid | ||||
| No. of cases | 28 | 28 | 38 | 24 |
| OR (95% CI) a | Ref. | 0.86 (0.44–1.67) | 1.02 (0.54–1.92) | 0.56 (0.27–1.13) |
| OR (95% CI) b | Ref. | 1.60 (0.64–3.99) | 1.54 (0.60–3.94) | 0.76 (0.27–2.13) |
| Hydroxyphenilacetic acid | ||||
| No. of cases | 17 | 16 | 47 | 38 |
| OR (95% CI) a | Ref. | 0.83 (0.38–1.82) | 2.62 (1.33–5.17) | 1.61 (0.79–3.28) |
| OR (95% CI) b | Ref. | 0.61 (0.24–1.58) | 1.66 (0.71–3.88) | 0.77 (0.29–2.07) |
| Caffeic acid | ||||
| No. of cases | 8 | 14 | 44 | 52 |
| OR (95% CI) a | Ref. | 1.42 (0.71–2.83) | 0.74 (0.39–1.43) | 0.28 (0.13–0.58) |
| OR (95% CI) b | Ref. | 1.86 (0.65–5.29) | 0.79 (0.30–2.08) | 0.32 (0.11–0.87) |
| Cinnamic acid | ||||
| No. of cases | 30 | 29 | 25 | 34 |
| OR (95% CI) a | Ref. | 0.87 (0.45–1.65) | 0.76 (0.39–1.48) | 0.81 (0.42–1.54) |
| OR (95% CI) b | Ref. | 1.29 (0.52–3.20) | 0.69 (0.28–1.73) | 1.06 (0.39–2.83) |
| Vanillic acid | ||||
| No. of cases | 5 | 24 | 53 | 36 |
| OR (95% CI) a | Ref. | 1.84 (0.92–3.67) | 0.99 (0.50–1.94) | 0.53 (0.25–1.11) |
| OR (95% CI) b | Ref. | 0.95 (0.33–2.73) | 0.54 (0.20–1.46) | 0.30 (0.10–0.85) |
| Ferulic acid | ||||
| No. of cases | 19 | 12 | 36 | 51 |
| OR (95% CI) a | Ref. | 1.51 (0.77–2.98) | 0.65 (0.33–1.25) | 0.50 (0.26–0.97) |
| OR (95% CI) b | Ref. | 1.62 (0.60–4.37) | 0.63 (0.25–1.60) | 0.44 (0.17–1.10) |
| Phenolic Acid Quartiles, OR (95% CI) a | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Total phenolic acids | Ref. | 0.75 (0.16–3.55) | 0.68 (0.14–3.21) | 0.34 (0.05–2.22) |
| Subclasses | ||||
| Hydroxybenzoic acids | Ref. | 0.52 (0.09–2.97) | 0.43 (0.50–0.09) | 0.85 (0.86–0.20) |
| Hydroxycinammic acid | Ref. | 0.44 (0.10–1.86) | 0.25 (0.05–1.32) | 0.10 (0.01–1.10) |
| Hydroxyphenilacetic acid | Ref. | 0.45 (0.04–5.18) | 3.40 (0.67–17.22) | 0.71 (0.09–5.66) |
| Individual phenolic acids | ||||
| Caffeic acid | Ref. | 0.62 (0.14–2.74) | 0.31 (0.07–1.33) | 0.01 (0.00–1.00) |
| Cinnamic acid | Ref. | 0.18 (0.02–1.61) | 0.57 (0.13–2.53) | 0.46 (0.10–2.15) |
| Vanillic acid | Ref. | 1.58 (0.35–7.11) | 0.69 (0.14–3.43) | 0.13 (0.01–1.55) |
| Ferulic acid | Ref. | 1.51 (0.32–1.10) | 1.66 (0.97–2.83) | 1.66 (0.97–2.83) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, G.I.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules 2017, 22, 2159. https://doi.org/10.3390/molecules22122159
Russo GI, Campisi D, Di Mauro M, Regis F, Reale G, Marranzano M, Ragusa R, Solinas T, Madonia M, Cimino S, et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules. 2017; 22(12):2159. https://doi.org/10.3390/molecules22122159
Chicago/Turabian StyleRusso, Giorgio Ivan, Daniele Campisi, Marina Di Mauro, Federica Regis, Giulio Reale, Marina Marranzano, Rosalia Ragusa, Tatiana Solinas, Massimo Madonia, Sebastiano Cimino, and et al. 2017. "Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy" Molecules 22, no. 12: 2159. https://doi.org/10.3390/molecules22122159