Trypanocidal Activity of Quinoxaline 1,4 Di-N-oxide Derivatives as Trypanothione Reductase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biological Activity In Vitro on Epimastigotes
2.2. Biological Activity In Vitro towards Trypomastigotes T. cruzi
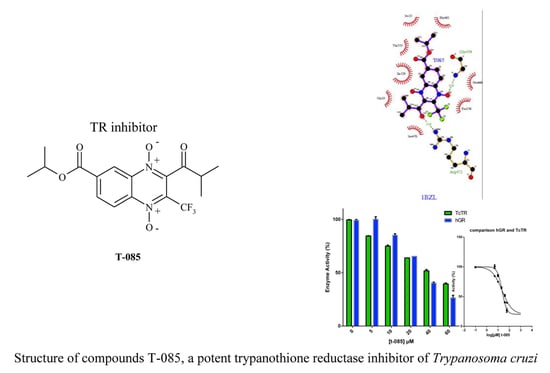
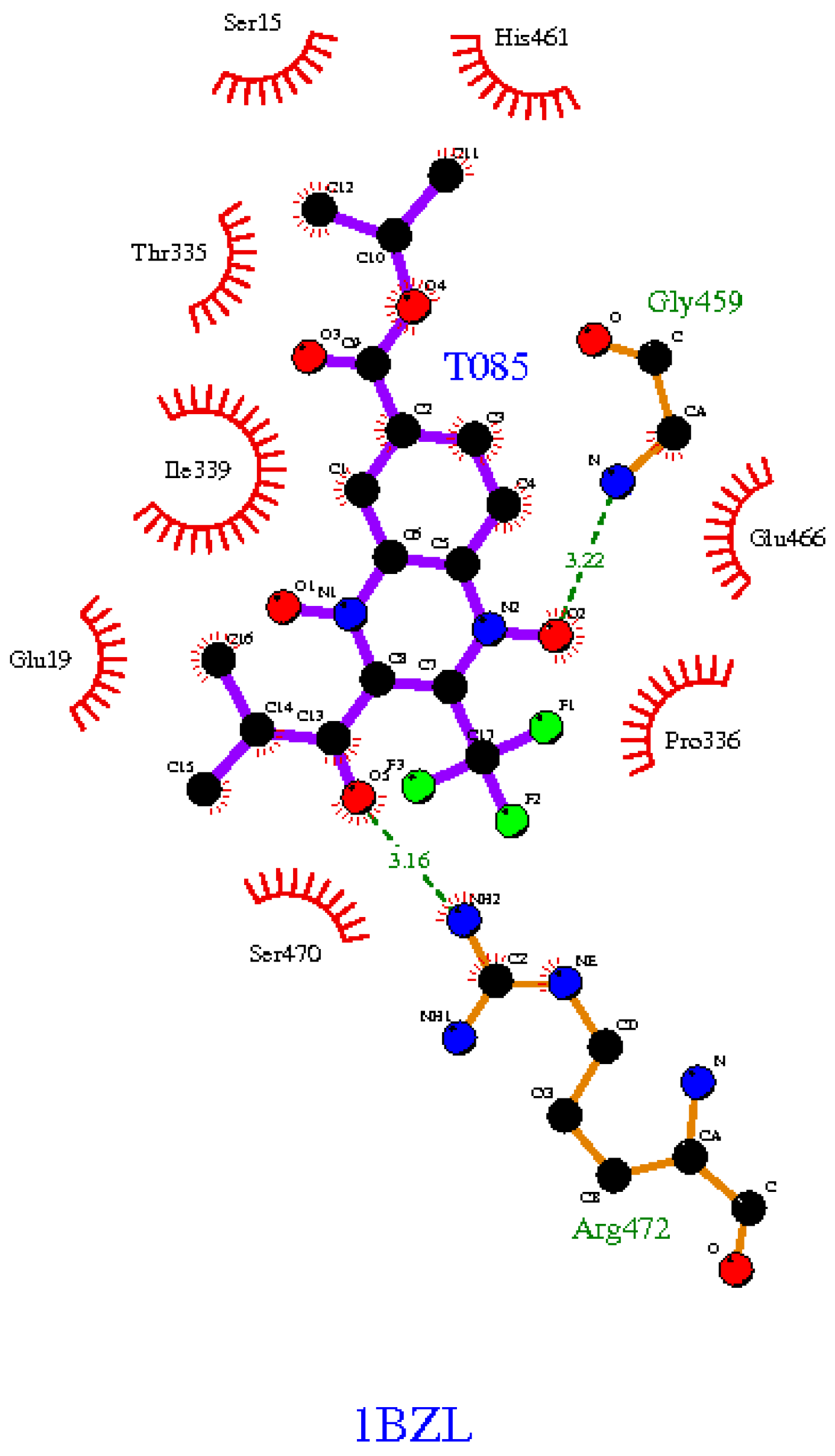
2.3. In Silico Binding Prediction
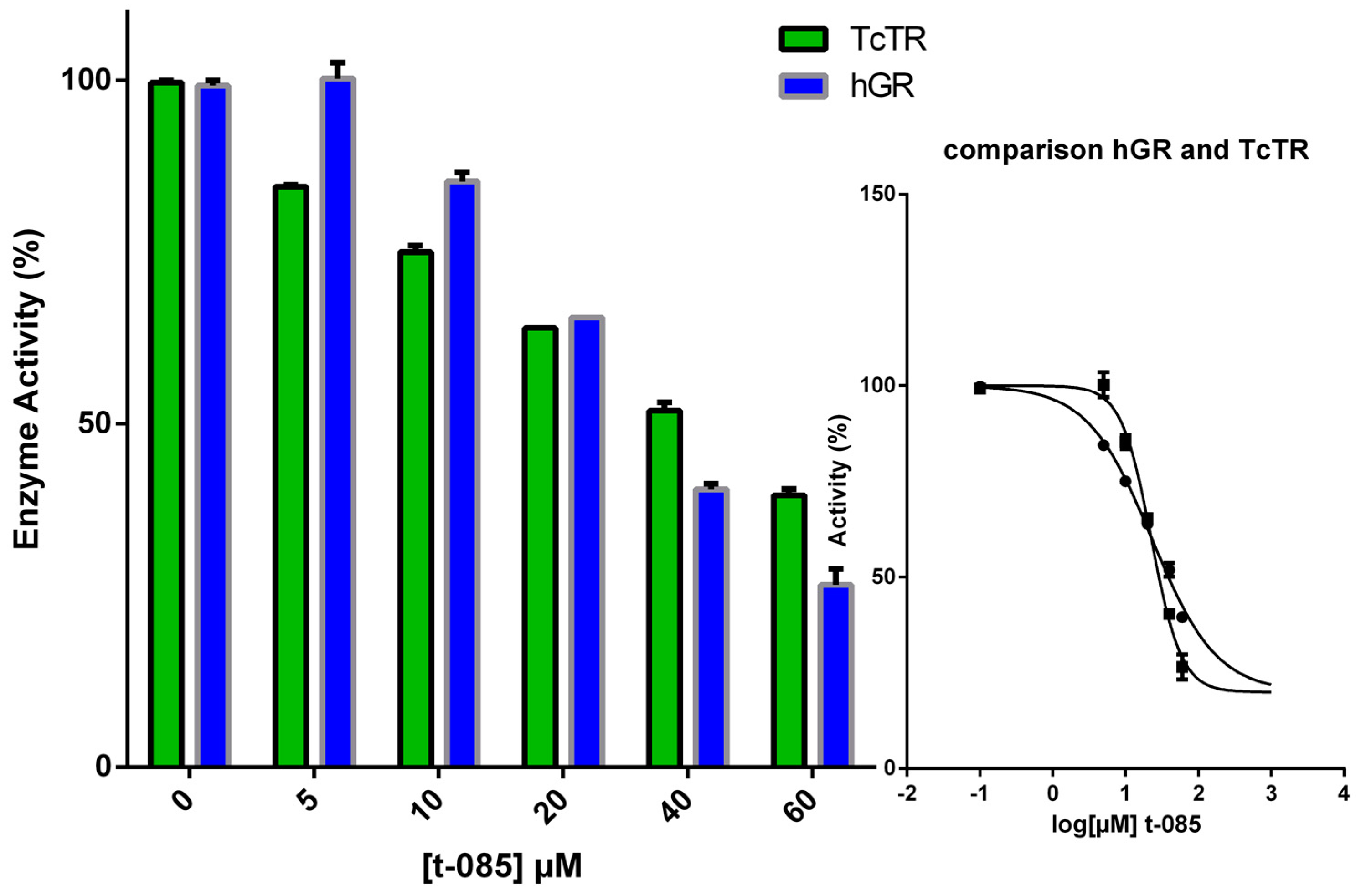
2.4. TR Inhibition
3. Materials and Methods
3.1. Synthetic Compounds
Chemical Compounds
3.2. Biological Assays
3.2.1. Parasites
3.2.2. Evaluation on Epimastigotes and IC50
3.2.3. Evaluation on Bloodstream Trypomastigotes and LC50
3.2.4. Cytotoxicity Assay and CC50
3.2.5. Determination of the SI and Selection of Active Compounds
3.3. Molecular Docking
3.4. Enzyme Inhibitor Studies
3.4.1. IC50 Determination
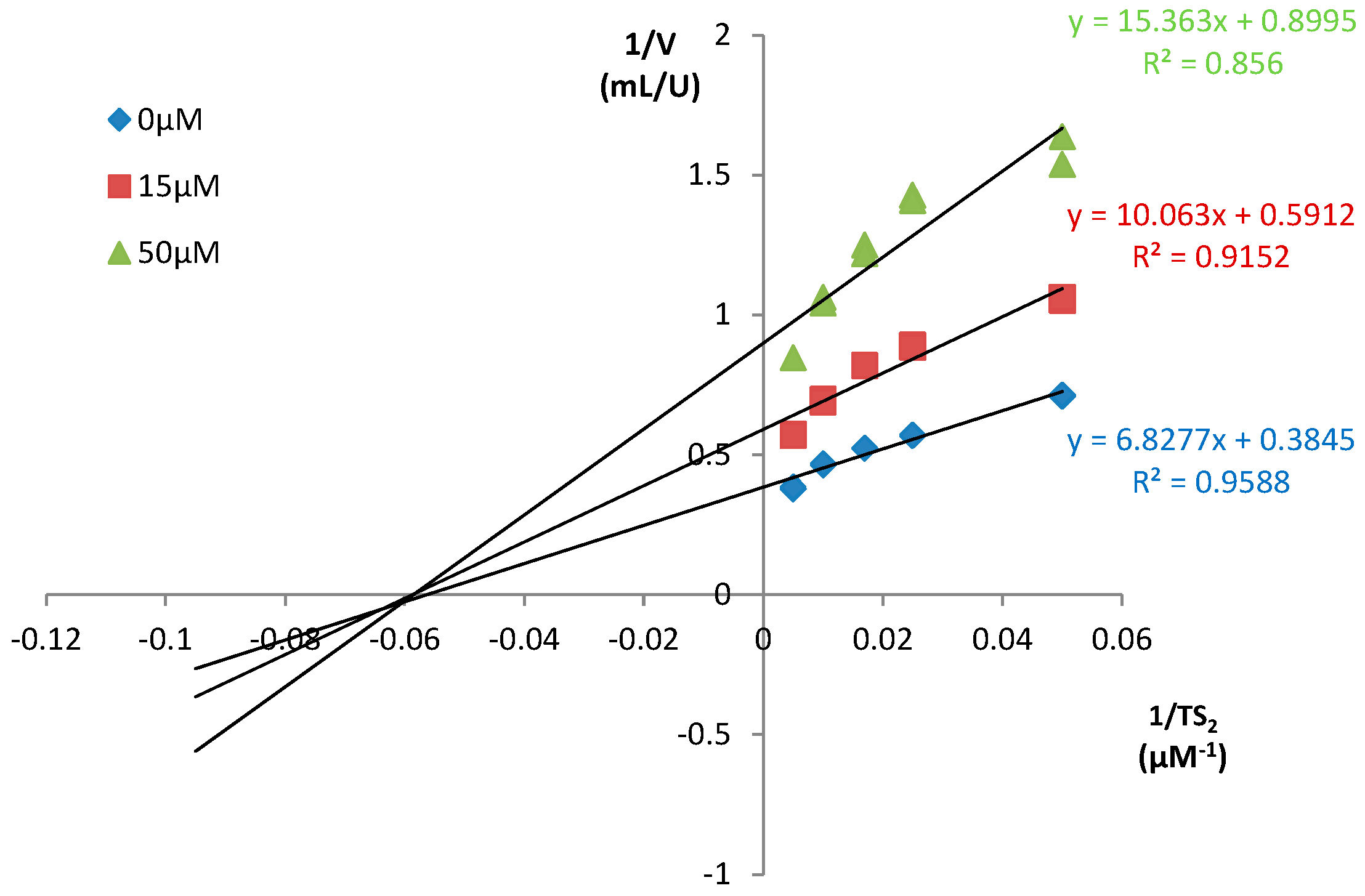
3.4.2. Determination of the Type of Inhibition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- Bern, C. Chagas disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [PubMed]
- Shikanai-Yasuda, M.A.; Barbosa Carvalho, N. Oral transmission of Chagas disease. Clin. Infec. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. In Neglected Tropical Diseases-Latin America and the Caribbean; Franco-Paredes, C., Santos-Preciado, J.I., Eds.; Springer: New York, NY, USA, 2015; pp. 45–72. [Google Scholar]
- World Health Organization, Chagas Disease (American Trypanosomiasis). Available online: http://www.who.int/mediacentre/factsheets/fs340/en/ (accessed on 10 October 2016).
- Nunes, M.C.; Dones, W.; Morillo, C.A.; Encina, J.J.; Ribeiro, A.L. Chagas Disease: An overview of clinical and epidemiological aspects. J. Am. Coll. Cardiol. 2013, 62, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Belaunzarán, M.L. Enfermedad de Chagas: Globalización y nuevas esperanzas para su cura. Rev. Argent. Microbiol. 2015, 47, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Sosa Estani, S.; Altcheh, J.; Riarte, A.; Freilij, H.; Fernandez, M.; Lloveras, S.; Pereiro, A.; Castellano, L.G.; Salvatella, R.; Nicholls, R.S. Lineamientos básicos del tratamiento etiológico de la enfermedad de Chagas. Medicina (B. Aires) 2015, 75, 270–272. [Google Scholar]
- Brunton, L.; Lazo, J.; Parker, K. Las Bases Farmacológicas de la Terapéutica: Goodman & Gilman, 11th ed.; McGraw-Hill Interamericana: Distrito Federal, Mexico, 2012; pp. 1052–1162. [Google Scholar]
- Murta, S.M.; Gazzinelli, R.T.; Brener, Z.; Romanha, A.J. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma. cruzi to benznidazole and nifurtimox. Mol. Biochem. Parasitol. 1998, 93, 203–214. [Google Scholar] [CrossRef]
- Mejía-Jaramillo, A.M.; Fernández, G.J.; Montilla, M.; Nicholls, R.S.; Triana-Chávez, O. Sensibilidad al benzonidazol de cepas de Trypanosoma. cruzi sugiere la circulación de cepas naturalmente resistentes en Colombia. Biomedica 2012, 32, 196–205. [Google Scholar]
- Mejía, A.M.; Hall, B.S.; Taylor, M.C.; Gómez-Palacio, A.; Wilkinson, S.R.; Triana-Chávez, O.; Kelly, J.M. Benznidazole-Resistance in Trypanosoma. cruzi is a readily acquired trait that can arise independently in a single Population. J. Infec. Dis. 2012, 206, 220–228. [Google Scholar]
- Guzmán-Marín, E.S.; Acosta-Viana, K.Y.; Jiménez-Coello, M. La Enfermedad de Chagas: Retos del tratamiento. Biomédica 2016, 27, 95–98. [Google Scholar]
- Vicente, E.; Pérez-Silanes, S.; Lima, L.M.; Ancizu, S.; Burguete, A.; Solano, B.; Villar, R.; Aldana, I.; Monge, A. Selective activity against Mycobacterium. tuberculosis of new quinoxaline 1,4-di-N-oxides. Bioorg. Med. Chem. 2009, 17, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Duque-Montano, B.E.; Gomez-Caro, L.C.; Sanchez-Sanchez, M.; Monge, A.; Hernandez-Baltazar, E.; Rivera, G.; Torres-Angeles, O. Synthesis and in vitro evaluation of new ethyl and methyl quinoxaline-7-carboxylate 1,4-di-N-oxide against Entamoeba histolytica. Bioorg. Med. Chem. 2013, 21, 4550–4558. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P.L.; Mollicotti, P.; Sechi, L.; Zanetti, S. Synthesis, anti-mycobacterial, anti-trichomonas and anti-candida in vitro activities of 2-substituted-6,7-difluoro-3-methyl quinoxaline 1,4-dioxides. Eur. J. Med. Chem. 2004, 39, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Moreira-Lima, L.; Solano, B.; Vicente, E.; Perez-Silanes, S.; Maurel, S.; Michel, S.; Aldana, I.; Monge, A.; Deharo, E. Antiplasmodial structure-activity relationship of 3-trifluoromethyl-2-arylcarbonyl quinoxaline1,4-di-N-oxide derivatives. Exp. Parasitol. 2008, 118, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Estevez, Y.; Quiliano, M.; Burguete, A.; Cabanillas, B.; Zimic, M.; Malaga, E.; Verástegui, M.; Pére-Silanes, S.; Aldana, I.; Castillo, D.; et al. Trypanocidal properties, structure-activity relationship and computational studies of quinoxaline 1,4-di-N-oxide derivatives. Exp. Parasitol. 2011, 127, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; di Maio, R.; González, M.; Risso, M.; Saenz, P.; Seoane, G.; Denicola, A.; Peluffo, G.; Quijano, C.; Olea-Azar, C. 1,2,5-Oxadiazole N-oxide derivatives and related compounds as potential antitrypanosomal drugs: structure-activity relationships. J. Med. Chem. 1999, 42, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.; Cerecetto, H.; di Maio, R.; González, M.; Alfaro, M.E.; Jaso, A.; Zarranz, B.; Ortega, M.A.; Aldana, I.; Monge-Vega, A. Quinoxaline N,N′-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi, structure-activity relationships. Bioorg. Med. Chem. 2004, 4, 3835–3839. [Google Scholar] [CrossRef] [PubMed]
- Ancizu, S.; Moreno, E.; Torres, E.; Burguete, A.; Pérez-Silanes, S.; Benítez, D.; Villar, R.; Solano, B.; Marín, A.; Aldana, I.; et al. Heterocyclic-2-carboxylic acid (3-cyano-1,4-di-N-oxidequinoxalin-2-Yl)amide derivatives as hits for the development of neglected disease drugs. Molecules 2009, 14, 2256–2272. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.; Cabrera, M.; Hernández, P.; Boiani, L.; Lavaggi, M.L.; di Maio, R.; Yaluff, G.; Serna, E.; Torres, S.; Ferreira, M.E.; et al. 3-Trifluoromethylquinoxaline N,N’-dioxides as anti-trypanosomatid agents. Identification of optimal anti-T. cruzi agents and mechanism of action studies. J. Med. Chem. 2011, 54, 3624–3636. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Moreno-Viguri, E.; Galiano, S.; Devarapally, G.; Crawford, P.W.; Azqueta, A.; Arbillaga, L.; Varela, J.; Birriel, E.; di Mairo, R.; et al. Novel Quinoxaline 1,4-di-N-oxide Derivatives as new potential antichagasic agents. Eur. J. Med. Chem. 2013, 66, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Rocha, J.C.; Sánchez-Torres, L.; Nogueda-Torres, B.; Segura-Cabrera, A.; García-Pérez, C.A.; Bocanegra-García, V.; Palos, I.; Monge, A.; Rivera, G. Anti-Trypanosoma cruzi and anti-leishmanial activity by quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives. Parasitol. Res. 2014, 113, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.E.; Romero, A. Grupo trifluorometilo: Un sustituyente importante en química medicinal. Rev. Fac. Farm. UCV 2011, 74, 40–52. [Google Scholar]
- Vega-Gomez, M.C.; Rolón, M.S.; Yaluff, G. Modelos de evaluación biológica in vitro e in vivo utilizados en la búsqueda de fármacos antichagásicos. In Enfermedad de Chagas: Estrategias en la búsqueda de nuevos medicamentos, una visión iberoamericana; Cerecetto-Meyer, H., González, M., Eds.; Ed Documaster, SA de CV: Distrito Federal, Mexico, 2012; pp. 267–297. [Google Scholar]
- Dos-Santos, V.A.F.F.; Leite, K.M.; da Costa-Siqueira, M.; Regasini, L.O.; Martinez, I.; Nogueira, C.T.; Kolos Galuppo, M.; Stolf, B.S.; Soares-Pereira, A.M.; Cicarelli, R.M.B.; et al. Antiprotozoal Activity of Quinonemethide Triterpenes from Maytenus ilicifolia (Celastraceae.). Molecules 2013, 18, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.S.; Zhang, Y.; Berriman, M.; Cunningham, M.L.; Fairlamb, A.H.; Hunter, W.N. Crystal structure of Trypanosoma. cruzi trypanothione reductase in complex with trypanothione, and the structure-based discovery of new natural product inhibitors. Structure 1999, 7, 81–89. [Google Scholar] [CrossRef]
- Laskowki, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Persch, E.; Bryson, S.; Todoroff, N.K.; Eberle, C.; Thelemann, J.; Dirdjaja, N.; Kaiser, M.; Weber, M.; Derbani, H.; Brun, R.; et al. Binding to large enzyme pockets: small-molecule inhibitors of trypanothione reductase. Chem. Med. Chem. 2014, 9, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Gómez Caro, L.C.; Sánchez Sánchez, M.; Bocanegra García, V.; Monge, A.; Rivera, G. Synthesis of quinoxaline 1,4-di-N-oxide derivatives on solid support using room temperature and microwave-assisted solvent-free procedures. Quim Nova 2011, 34, 1147–1151. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Bosseno, M.F.; Barnabé, C.; Gastelum, E.M.; Kasten, F.L.; Ramsey, J.; Espinoza, B.; Breniere, F. Predominance of Trypanosoma cruzi Lineage I in Mexico. J. Clin. Microbiol. 2002, 40, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Sánchez, R.; León, M.P.; Matta, V.; Reyes, P.A.; López, R.; Jay, D.; Monteón, V.M. Trypanosoma cruzi isolates from Mexican and Guatemalan acute and chronic chagasic cardiopathy patients belong to Trypanosoma cruzi I. Mem. Inst. Oswaldo Cruz 2005, 100, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.T.; Silva-Jardim, I.; Portapilla, G.B.; de Lima, G.M.; Costa, F.C.; Anibal, F.F.; Thieman, O.H. In vivo and in vitro auranofin activity against Trypanosoma cruzi: Possible new uses for an old drug. Exp. Parasitol. 2016, 166, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Cotinguiba, F.; Regasini, L.O.; da Silva Bolzani, V.; Debonsi, H.M.; Duó Passerini, G.; Barretto Cicarelli, R.M.; Kato, M.J.; Furlan, M. Piperamides and their derivatives as potential anti-trypanosomal agents. Med. Chem. Res. 2009, 18, 703–711. [Google Scholar] [CrossRef]
- Muelas-Serrano, S.; Nogal-Ruiz, J.J.; Gómez-Barrio, A. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi. Parasitol. Res. 2000, 86, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop Sao Paulo 1962, 4, 389–396. [Google Scholar] [PubMed]
- Díaz-Chiguer, D.L.; Márquez-Navarro, A.; Nogueda-Torres, B.; León-Ávila, G.L.; Pérez-Villanueva, J.; Hernández-Campos, A.; Castillo, R.; Ambrosio, J.R.; Nieto-Meneses, R.; Yépez-Mulia, L.; et al. In vitro and in vivo trypanocidal activity of some benzimidazole derivatives against two strains of Trypanosoma cruzi. Acta Trop 2012, 122, 108–112. [Google Scholar] [CrossRef] [PubMed]
- O’Brian, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar]
- Mahmoudvand, H.; Tavakoli, R.; Sharififar, F.; Minaie, K.; Ezatpour, B.; Jahanbakhsh, S.; Sharifi, I. Leishmanicidal and cytotoxic activities of Nigella sativa and its active principle, thymoquinone. Pharm. Biol. 2015, 53, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Nunes Dos Santos, R.A.; Batista, J.; Rosa, S.I.; Torquato, H.F.; Bassi, C.L.; Ribeiro, T.A.; de Sousa, P.T.; Beserra, A.M.; Fontes, C.J.; da Silva, L.E.; et al. Leishmanicidal effect of Spiranthera odoratíssima (Rutaceae) and its isolated alkaloid skimmianine occurs by a nitric oxide dependent mechanism. Parasitology 2011, 138, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comp. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team 2016 R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org. (accessed on 15 October 2016).
- Sullivan, F.X.; Walsh, C.T. Cloning, sequencing, overproduction and purification of trypanothione reductase from T. cruzi. Mol. Biochem. Parasit. 1999, 44, 145–147. [Google Scholar] [CrossRef]
- Comini, M.A.; Dirdjaja, N.; Kaschel, M.; Krauth-Siegel, R.L. Preparative enzymatic synthesis of trypanothione and trypanothione analogues. Int. J. Parasitol. 2009, 39, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Jockers-Scherübl, M.C.; Schirmer, R.H.; Krauth-Siegel, R.L. Trypanothione reductase from Trypanosoma. cruzi. Catalytic properties of the enzyme and inhibition studies with trypanocidal compounds. Eur. J. Biochem. 1989, 180, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kemmer, G.; Keller, S. Nonlinear least-squares data fitting in Excel spreadsheets. Nat. Protoc. 2010, 5, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput. Meth. Prog. Bio. 2001, 65, 191–200. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds T-064–T-130 are available from the authors.
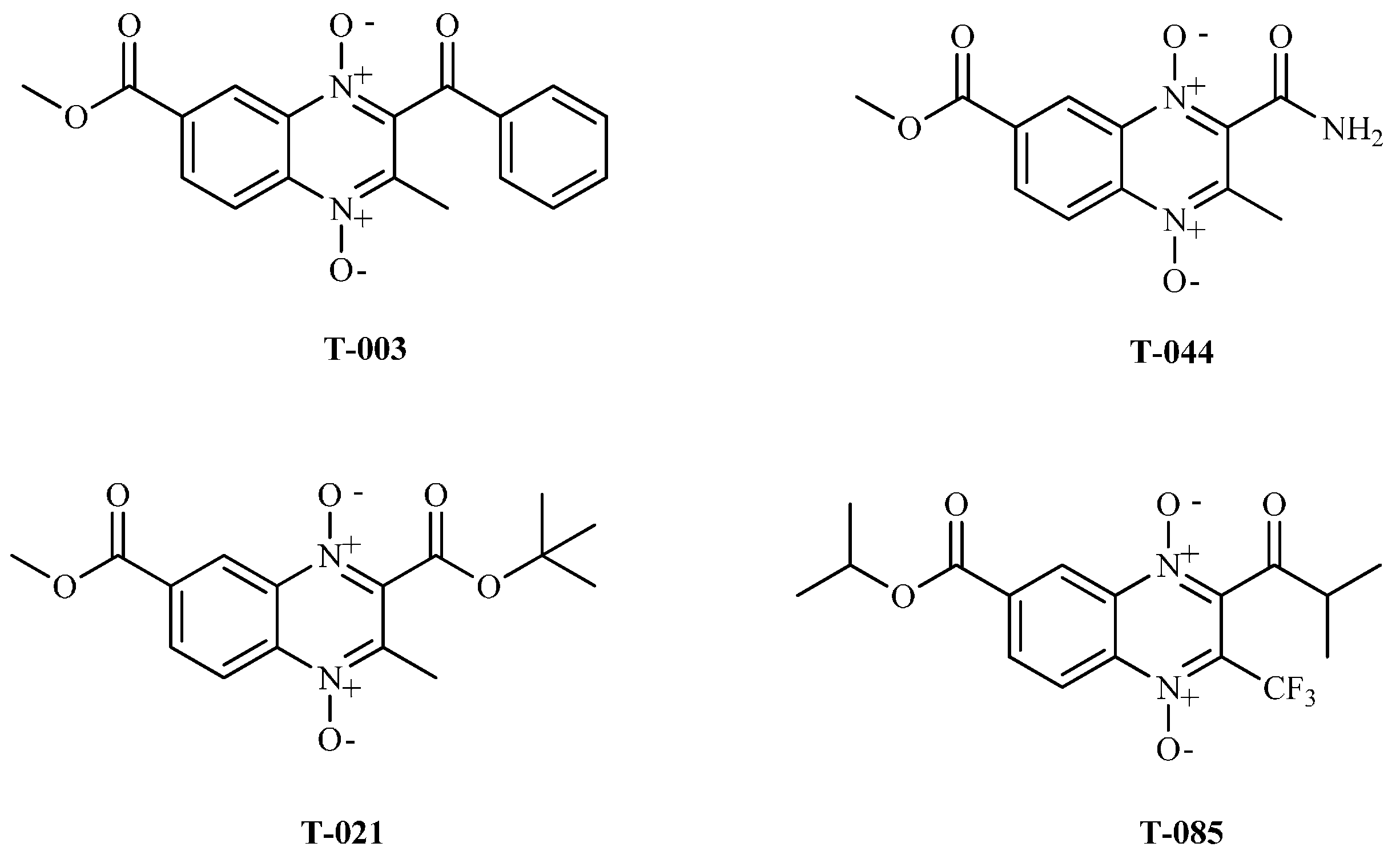
| Compound | R2 | R3 | R7 | IC50 (µM) | CC50 (µM) | SI |
|---|---|---|---|---|---|---|
| Bnz | 42.34 ± 5.76 | 352.01 ± 17.1 | 8.31 | |||
| Nfx | 8.74 ± 3.8 | 201.05 ± 12.5 | 22.98 | |||
| T-064 | COOCH3 | CH3 | (CH3)2CH | 43.66 ± 8.6 | 135.04 ± 11.1 | 3.09 |
| T-065 | COOCH2CH3 | CH3 | (CH3)2CH | >147.86 | >295.73 | Nd a |
| T-066 | COOC(CH3)3 | CH3 | (CH3)2CH | >136.55 | >273.09 | Nd a |
| T-067 | COOCH2CH3 | CH2COOCH2CH3 | (CH3)2CH | 12.12 ± 0.4 | 44.37 ± 7.1 | 3.66 |
| T-069 | COC4H3S | CF3 | (CH3)2CH | 4.95 ± 0.5 | 12.80 ± 0.6 | 2.58 |
| T-070 | COCH3 | CF3 | (CH3)2CH | 2.83 ± 0.7 | 17.50 ± 0.7 | 6.17 |
| T-071 | COC6H5 | CF3 | (CH3)2CH | 4.03 ± 0.5 | 6.70 ± 0.8 | 1.66 |
| T-072 | COC10H7 | CF3 | (CH3)2CH | 46.51 ± 7.5 | 15.14 ± 0.6 | 0.32 |
| T-073 | COC4H3O | CF3 | (CH3)2CH | 33.66 ± 9.2 | 55.35 ± 2.9 | 1.64 |
| T-085 | COCH(CH3)2 | CF3 | (CH3)2CH | 2.42 ± 0.5 | 10.38 ± 0.8 | 4.28 |
| T-088 | COOCH3 | CH3 | CH3CH2CH2 | 138.55 ± 13.5 | 143.00 ± 9.7 | 1.03 |
| T-089 | COC4H3S | CF3 | CH3CH2CH2 | 7.59 ± 1.4 | 7.71 ± 0.7 | 1.01 |
| T-090 | COOCH2CH3 | CH3 | CH3CH2CH2 | 155.14 ± 12.3 | 145.50 ± 9.2 | 0.93 |
| T-091 | COOC(CH3)3 | CH3 | CH3CH2CH2 | >136.55 | >273.09 | Nd a |
| T-097 | CONHC6H5 | CH3 | (CH3)2CH | >130.50 | >261.00 | Nd a |
| T-098 | COC6H5 | CH3 | (CH3)2CH | 141.90 ± 8.3 | 139.62 ± 8.1 | 0.98 |
| T-107 | COC(CH3)3 | C(CH3)3 | (CH3)2CH | 91.71 ± 10.5 | 164.43 ± 7.7 | 1.79 |
| T-108 | CONH2 | CH3 | (CH3)2CH | 130.67 ± 12.3 | 221.35 ± 9.4 | 1.69 |
| T-116 | COOCH2CH3 | CF3 | (CH3)2CH | 5.20 ± 2.1 | 14.51 ± 0.8 | 2.78 |
| T-117 | COC(CH3)3 | CF2CF2CF3 | (CH3)2CH | 100.03 ± 7.9 | 138.89 ± 5.6 | 1.38 |
| T-118 | COCF2CF3 | CF3 | (CH3)2CH | 72.17 ± 8.0 | 157.39 ± 6.3 | 2.18 |
| T-124 | COOCH2CH3 | CF3 | CH3CH2CH2 | 4.21 ± 0.5 | 8.77 ± 3.4 | 2.08 |
| T-125 | CONHC6H5 | CH3 | CH3CH2CH2 | >130.50 | >261.00 | Nd a |
| T-126 | COCH3 | CH3 | CH3CH2CH2 | >163.33 | >326.67 | Nd a |
| T-130 | CONH2 | CH3 | CH3CH2CH2 | 238.28 ± 11.5 | >325.61 | Nd a |
| Compound | % Lysis at 50 μg/mL | LC50 (µM) | ||
|---|---|---|---|---|
| NINOA Strain | INC-5 Strain | NINOA Strain | INC-5 Strain | |
| Bzn | 58.10 ± 4.5 | 53.50 ± 5.6 | 98.83.72 ± 17.7 | 85.26 ± 16.7 |
| Nfx | 67.30 ± 5.4 | 62.60 ± 3.9 | 117.34 ± 17.7 | 113.86 + 17.8 |
| T-064 | 3.20 ± 2.0 | 1.70 ± 2.0 | Nd a | Nd a |
| T-065 | 0.77 ± 1.58 | 3.78 ± 5.7 | Nd a | Nd a |
| T-066 | 2.60 ± 4.3 | 5.40 ± 3.7 | Nd a | Nd a |
| T-067 | 18.50 ± 2.2 | 14.38 ± 9.2 | Nd a | Nd a |
| T-069 | 57.33 ± 2.8 | 77.74 ± 9.3 | 98.03 ± 6.1 | 110.8 ± 9.3 |
| T-070 | 45.97 ± 2.7 | 10.82 ± 5.4 | Nd a | Nd a |
| T-071 | 58.79 ± 4.4 | 69.18 ± 5.1 | 97.8 ± 6.3 | 103.2 ± 8.3 |
| T-072 | 17.77 ± 2.3 | 4.11 ± 3.8 | Nd a | Nd a |
| T-073 | 35.05 ± 4.5 | 22.10 ± 4.32 | Nd a | Nd a |
| T-085 | 74.17 ± 6.4 | 76.20 ± 1.2 | 59.9 ± 7.9 | 73.1 ± 12.4 |
| T-088 | 16.26 ± 11.1 | 33.56 ± 6.8 | Nd a | Nd a |
| T-089 | 51.65 ± 3.4 | 41.98 ± 6.5 | 114.7 ± 8.4 | 122.1 ± 6.5 |
| T-090 | 28.57 ± 3.1 | 47.26 ± 3.3 | Nd a | Nd a |
| T-091 | 7.30 ± 3.3 | 8.15 ± 2.2 | Nd a | Nd a |
| T-097 | 12.18 ± 10.9 | 12.37 ± 5.2 | Nd a | Nd a |
| T-098 | 6.89 ± 5.8 | 0.34 ± 0.6 | Nd a | Nd a |
| T-107 | 6.28 ± 4.7 | 5.32 ± 3.8 | Nd a | Nd a |
| T-108 | 12.80 ± 7.2 | 18.80 ± 2.8 | Nd a | Nd a |
| T-116 | 49.88 ± 4.0 | 17.64 ± 2.6 | Nd a | Nd a |
| T-117 | 40.51 ± 4.1 | 10.31 ± 4.9 | Nd a | Nd a |
| T-118 | 34.39 ± 3.1 | 0.00 ± 3.2 | Nd a | Nd a |
| T-124 | 24.61 ± 4.3 | 13.40 ± 8.9 | Nd a | Nd a |
| T-125 | 35.62 ± 3.6 | 0.00 ± 1.4 | Nd a | Nd a |
| T-126 | 17.69 ± 3.1 | 0.00 ± 6.1 | Nd a | Nd a |
| T-130 | 4.65 ± 3.4 | 0.69 ± 1.2 | Nd a | Nd a |
| Compound | Vina Score | MW |
|---|---|---|
| T-072 | −8.6 | 472.124 |
| T-097 | −7.7 | 383.148 |
| T-098 | −7.7 | 368.137 |
| T-071 | −7.6 | 422.108 |
| T-073 | −7.4 | 412.088 |
| T-069 | −7.3 | 410.168 |
| T-003 | −7.2 | 340.105 |
| T-117 | −7.2 | 502.133 |
| T-118 | −7.2 | 464.061 |
| T-125 | −7.2 | 383.148 |
| T-070 | −7.1 | 360.093 |
| T-085 | −7.1 | 388.124 |
| T-089 | −6.9 | 428.065 |
| Trypanothionine | −6.9 | 721.288 |
| T-107 | −6.7 | 391.223 |
| T-108 | −6.7 | 307.116 |
| T-116 | −6.7 | 392.119 |
| T-066 | −6.6 | 366.179 |
| T-021 | −6.5 | 322.152 |
| T-044 | −6.5 | 279.085 |
| T-130 | −6.5 | 307.116 |
| T-045 | −6.4 | 293.101 |
| T-126 | −6.4 | 306.121 |
| T-064 | −6.2 | 324.132 |
| T-067 | −6.2 | 410.168 |
| T-065 | −6.1 | 338.147 |
| T-090 | −6.1 | 338.147 |
| T-091 | −6.1 | 366.179 |
| T-124 | −6.1 | 392.119 |
| T-088 | −5.9 | 324.132 |
| Compound | Inhibitor [µM] | % Inhibition of TR at | |
|---|---|---|---|
| 100 µM [TS2] | 40 µM [TS2] | ||
| T-085 | 5 | 15.2 | 20.3 |
| 10 | 24.7 | 34.1 | |
| 20 | 36.1 | 45.7 | |
| 40 | 48.1 | 55.1 | |
| 60 | 60.1 | 60.9 | |
| 80 * | 33 | 44 | |
| T-003 | 20 | 6 | 6.6 |
| 40 | 10 | 9 | |
| 60 | 12 | 14.8 | |
| 80 | 18 | 20.5 | |
| 100 | 19.3 | 23.8 | |
| T-021 | 40 | 0 | 0 |
| 100 | 0 | 7.5 | |
| T-044 | 40 | 0 | 0 |
| 100 | 0 | 0 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Vargas, K.F.; Nogueda-Torres, B.; Sánchez-Torres, L.E.; Suarez-Contreras, E.; Villalobos-Rocha, J.C.; Torres-Martinez, Y.; Lara-Ramirez, E.E.; Fiorani, G.; Krauth-Siegel, R.L.; Bolognesi, M.L.; et al. Trypanocidal Activity of Quinoxaline 1,4 Di-N-oxide Derivatives as Trypanothione Reductase Inhibitors. Molecules 2017, 22, 220. https://doi.org/10.3390/molecules22020220
Chacón-Vargas KF, Nogueda-Torres B, Sánchez-Torres LE, Suarez-Contreras E, Villalobos-Rocha JC, Torres-Martinez Y, Lara-Ramirez EE, Fiorani G, Krauth-Siegel RL, Bolognesi ML, et al. Trypanocidal Activity of Quinoxaline 1,4 Di-N-oxide Derivatives as Trypanothione Reductase Inhibitors. Molecules. 2017; 22(2):220. https://doi.org/10.3390/molecules22020220
Chicago/Turabian StyleChacón-Vargas, Karla Fabiola, Benjamin Nogueda-Torres, Luvia E. Sánchez-Torres, Erick Suarez-Contreras, Juan Carlos Villalobos-Rocha, Yuridia Torres-Martinez, Edgar E. Lara-Ramirez, Giulia Fiorani, R. Luise Krauth-Siegel, Maria Laura Bolognesi, and et al. 2017. "Trypanocidal Activity of Quinoxaline 1,4 Di-N-oxide Derivatives as Trypanothione Reductase Inhibitors" Molecules 22, no. 2: 220. https://doi.org/10.3390/molecules22020220