Cytotoxicity and Antioxidant Potential of Novel 2-(2-((1H-indol-5yl)methylene)-hydrazinyl)-thiazole Derivatives
Abstract
:1. Introduction
2. Results and Discussion
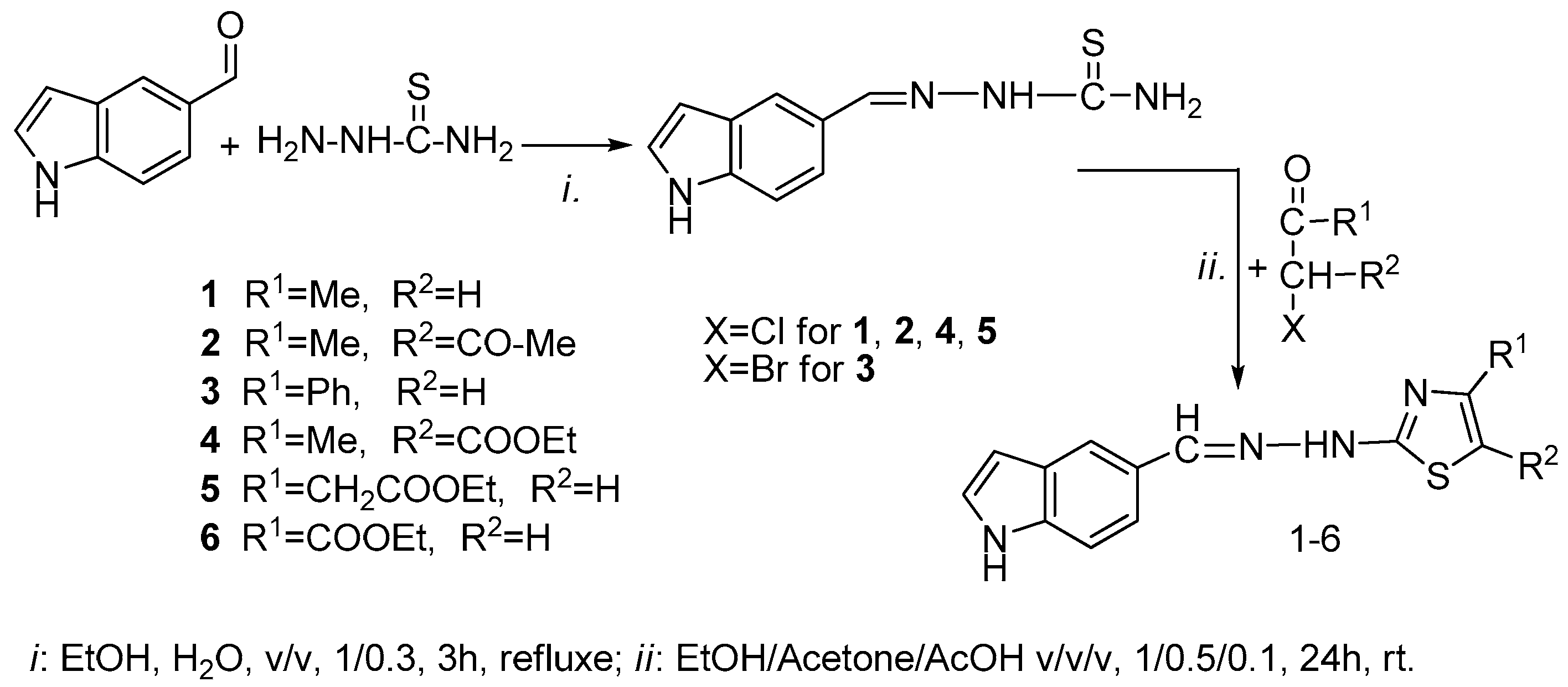
2.1. Synthesis of the New 2-(2-((1H-Indol-5-yl)methylene)hydrazinyl)thiazole Derivatives
2.2. Cytotoxic Activity
2.3. Antioxidant Activity
2.4. Computational Study
3. Experimental Section
3.1. Materials and Methods
3.2. Chemistry
3.2.1. General Procedure for (E)-2-[(1H-Indol-3-yl) methylene]thiosemicarbazone
3.2.2. General Procedure for Compounds 1–6






3.3. Biology
3.3.1. Cell Cultures
Cell Proliferation Inhibition
Statistical Analysis
3.3.2. Antioxidant Activity
DPPH Based Free Radical Scavenging Activity
Reducing Power by Ferric Reducing Antioxidant Power (FRAP) Test
Electron Paramagnetic Resonance (EPR) Spectroscopy Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Browne, L.M.; Conn, K.L.; Ayert, W.A.; Tewari, J.P. The camalexins: New phytoalexins produced in the leaves of camelinasativa (cruciferae). Tetrahedron 1991, 47, 3909–3914. [Google Scholar] [CrossRef]
- Glawischnig, E. Camalexin. Phytochemistry 2007, 68, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbno, A.; Spanò, V.; Sbarra, S.; Doldi, V.; de Cesare, M.; et al. Novel 1H-pyrrolo[2,3-b]pyridine derivatives nortopsentin analogues: Synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Parrino, B.; Lopergolo, A.; Pennati, M.; Zaffaroni, N.; Cirrincione, G. Synthesis and antitumor activity of 3-(2-phenyl-1,3-thiazol-4-yl)-1H-indoles and 3-(2-phenyl-1,3-thiazol-4-yl)-1H-7-azaindoles. ChemMedChem 2011, 6, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Meleddu, R.; Distinto, S.; Corona, A.; Bianco, G.; Cannas, V.; Esposito, F.; Artese, A.; Alcaro, S.; Matyus, P.; Bogdan, D.; et al. (3Z)-3-(2-[4-(aryl)-1,3-thiazol-2-yl]hydrazin-1-ylidene)-2,3-dihydro-1H-indol-2-one derivatives as dual inhibitors of HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2015, 93, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Kankanala, R.; Prakash, A.; Kannan, T. 2-(2-Hydrazinyl)thiazole derivatives: Design, synthesis and in vitro antimycobacterial studies. Eur. J. Med. Chem. 2013, 69, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Kondrasenko, A.A.; Goncharov, E.V.; Dugaev, K.P.; Rubaylo, A.I. CBL-2201. Report on a new designer drug: Napht-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate. Forensic Sci. Int. 2015, 257, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Cappadone, C.; Stefanelli, C.; Malucelli, E.; Zini, M.; Onofrillo, C.; Locatelli, A.; Rambaldi, M.; Sargenti, A.; Merolle, L.; Farruggia, G.; et al. p53-dependent and p53-independent anticancer activity of a new indole derivative in human osteosarcoma cells. Biochem. Biophys. Res. Commun. 2015, 467, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Estevão, M.S.; Carvalho, L.C.; Ribeiro, D.; Couto, D.; Freitas, M.; Gomes, A.; Fernandes, L.M.F.E.; Marques, M.M.B. Antioxidant activity of unexplored indole derivatives: Synthesis and screening. Eur. J. Med. Chem. 2010, 45, 4869–4878. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Rouf, A.; Tanyeli, C. Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 97, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Bolasco, A.; Secci, D.; Chimenti, A.; Granese, A.; Carradori, S.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S. Investigations on the 2-thiazolylhydrazyne scaffold: Synthesis and molecular modeling of selective human monoamine oxidase inhibitors. Bioorg. Med. Chem. 2010, 18, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
- Nosrat, O.; Mahmoodi, N.O.; Khalili, B.; Rezaeianzade, O.; Ghavidast, A. One-pot multicomponent synthesis of indol-3-ylhydrazinyl thiazoles as antimicrobial agents. Res. Chem. Intermed. 2016, 42, 6531–6542. [Google Scholar]
- Carradori, S.; Rotili, D.; de Monte, C.; Lenoci, A.; D’Ascenzio, M.; Rodriguez, V.; Filetici, P.; Miceli, M.; Nebbioso, A.; Altucci, L.; et al. Evaluation of a large library of (thiazol-2-yl)hydrazones and analogues as histone acetyltransferase inhibitors: Enzyme and cellular studies. Eur. J. Med. Chem. 2014, 80, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, C.M.; Oniga, O.; Parvu, A.; Tiperciuc, B.; Verite, P.; Pirnau, A.; Crisan, O.; Bojita, M.; Pop, P. Synthesis and anti-inflammatory evaluation of some new acyl-hydrazones bearing 2-aryl-thiazole. Eur. J. Med. Chem. 2011, 46, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Grozav, A.; Gaina, L.I.; Pileczki, V.; Crisan, O.; Silaghi-Dumitrescu, L.; Therrien, B.; Zaharia, V.; Berindan-Neagoe, I. The Synthesis and Antiproliferative Activities of New Arylidene-Hydrazinyl-Thiazole Derivatives. Int. J. Mol. Sci. 2014, 15, 22059–22072. [Google Scholar] [CrossRef] [PubMed]
- Ignat, A.; Lovasz, T.; Vasilescu, M.; Fischer-Fodor, E.; Tatomir, C.B.; Cristea, C.; Silaghi-Dumitrescu, L.; Zaharia, V. Heterocycles 27. Microwave Assisted Synthesis and Antitumour Activity of Novel Phenothiazinyl-Thiazolyl-Hydrazine Derivatives. Arch. Pharm. Chem. Life Sci. 2012, 345, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, V.; Ignat, A.; Palibroda, N.; Ngameni, B.; Kuete, V.; Fokunang, C.N.; Moungang, M.L.; Ngadjui, B.T. Synthesis of some p-toluenesulfonyl-hydrazinothiazoles and hydrazino-bisthiazoles and their anticancer activity. Eur. J. Med. Chem. 2010, 45, 5080–5085. [Google Scholar] [CrossRef]
- Spartan’06. Wavefunction, Inc.: Irvine, CA, USA. Available online: http://www.wavefun.com/ (accessed on 20 September 2016).
- Hehre, W.J. A Guide to Molecular Mechanics and Quantum Chemical Calculations; Wavefunction, Inc.: Irvine, CA, USA, 2003. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabulowski, C.F.; Frisch, M. Ab initio calculation of vibrational absorbtion and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623. [Google Scholar] [CrossRef]
- Yi, W.; Dubois, C.; Yahiaoui, S.; Haudecoeur, R.; Belle, C.; Song, H.; Hardre, R.; Reglier, M.; Boumendjel, A. Refinement of arylthiosemicarbazone pharmacophore in inhibition of mushroom tyrosinase. Eur. J. Med. Chem. 2011, 46, 4330–4335. [Google Scholar] [CrossRef]
- Nastasă, C.; Tiperciuc, B.; Duma, M.; Benedec, D.; Oniga, O. New hydrazones bearing thiazole scaffold: Synthesis, characterization, antimicrobial, and antioxidant investigation. Molecules 2015, 20, 17325–17338. [Google Scholar]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables-evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, M.; Olea-Azar, C.; Speisky, H.; Rodríguez, J. Determination of reactions between free radicals and selected Chilean wines and transition metals by ESR and UV–vis technique. J. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Crisan, G.; Vlase, L.; Crisan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.-M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative Studies on Polyphenolic Composition, Antioxidant and Antimicrobial Activities of Schisandrachinensis Leaves and Fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–6 are available from the authors A.G. anl L.I.G.
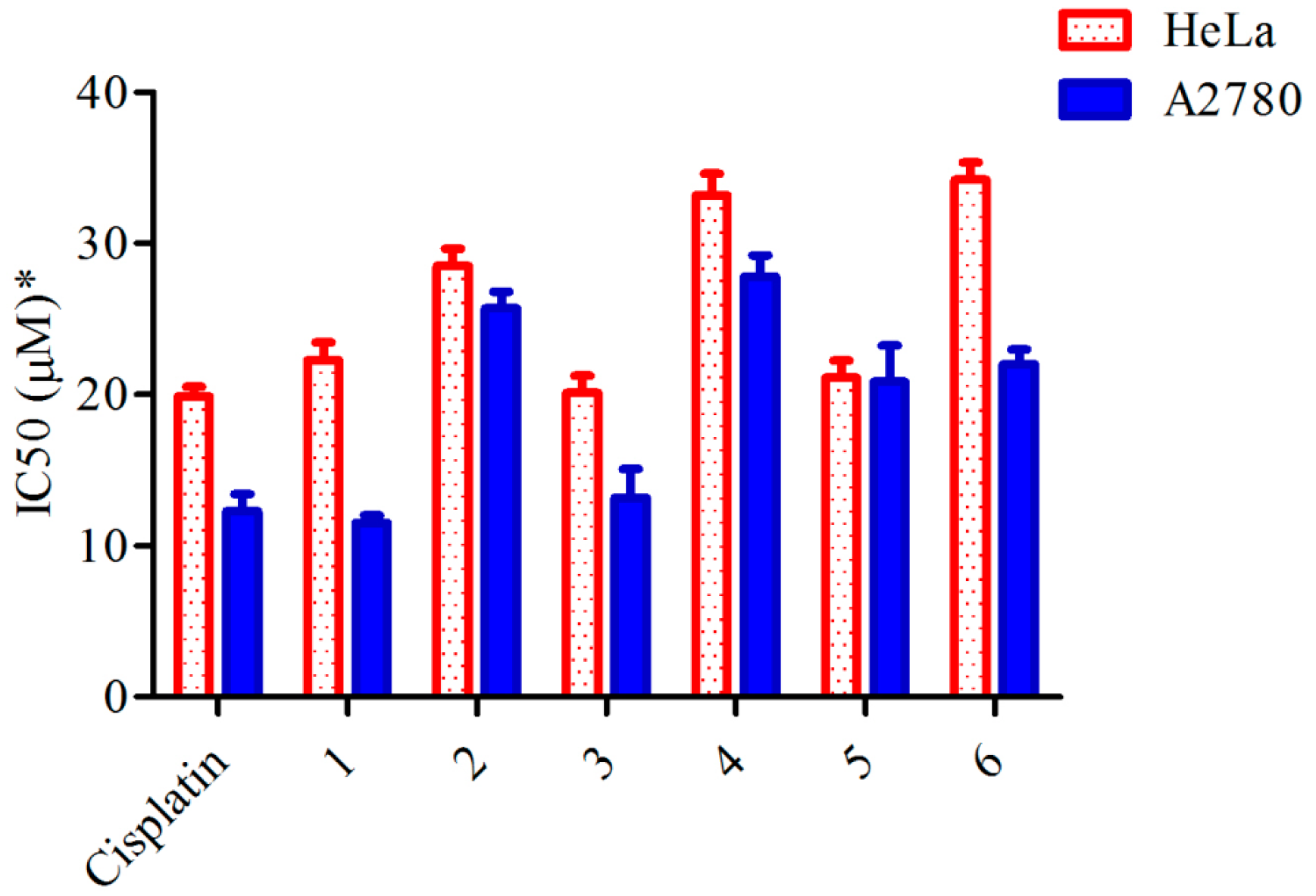
| Compounds | IC50 (μM) | |
|---|---|---|
| HeLa | A2780 | |
| Cisplatin | 20.10 ± 0.22 | 12.30 ± 0.22 |
| 1 | 22.42 ± 0.24 | 11.65 ± 0.21 |
| 2 | 28.55 ± 0.25 | 26.49 ± 0.21 |
| 3 | 19.42 ± 0.22 | 12.99 ± 0.50 |
| 4 | 33.98 ± 0.31 | 28.08 ± 0.23 |
| 5 | 19.84 ± 0.33 | 19.66 ± 0.50 |
| 6 | 34.72 ± 0.25 | 20.15 ± 0.26 |

| Compounds | Methods | ||
|---|---|---|---|
| DPPH IC 50 (µg/mL) | FRAP µM ET/g | EPR Integral Intensity Value | |
| 1 | 5.377 ± 0.52 | 9835.5518 ± 324 | 17 ± 0.14 |
| 2 | 11.850 ± 0.72 | 872.5288 ± 27 | 72 ± 0.23 |
| 3 | 9.131 ± 0.61 | 8176.5278 ± 298 | 24 ± 0.17 |
| 4 | 13.357 ± 0.68 | 6129.6298 ± 280 | 114 ± 0.3 |
| 5 | 12.974 ± 0.58 | 7431.0798 ± 263 | 82 ± 0.52 |
| 6 | 15.658 ± 0.63 | 3572.2890 ± 124 | 142 ± 0.34 |
| Trolox | 9.74 ± 0.24 | - | - |
| Ac.ascorbic | 2.46 ± 0.91 | - | - |
| Compounds | EHOMO (eV) | ELUMO (eV) | EHOMO-LUMO (eV) | HOMO Surface |
|---|---|---|---|---|
| 1 | −4.96 | −0.98 | 3.98 |  |
| 2 | −5.29 | −1.52 | 3.77 |  |
| 3 | −5.00 | −1.10 | 3.90 |  |
| 4 | −5.22 | −1.38 | 3.84 |  |
| 5 | −4.96 | −0.96 | 4.00 |  |
| 6 | −5.23 | −1.20 | 4.03 |  |
| Radical | E (kcal/mol) | EHOMO (eV) Hradical | ELUMO (eV) Hradical | Spin Density Map for Hradical | Spin Density on C5 for Hradical (eV) * | SOMO Orbital for Hradical | |
|---|---|---|---|---|---|---|---|
| 1 | H | −695,328.05 | −7.94 | 3.09 |  | 0.035 |  |
| I | −695,310.80 | ||||||
| 2 | H | −790,033.74 | −8.08 | 2.54 |  | 0.036 |  |
| I | −790,016.36 | ||||||
| 3 | H | −814,214.89 | −7.98 | 2.91 |  | 0.037 |  |
| I | −814,185.61 | ||||||
| 4 | H | −861,114.17 | −8.06 | 2.59 |  | 0.036 |  |
| I | −861,098.95 | ||||||
| 5 | H | −861,108.82 | −7.97 | 2.98 |  | 0.038 |  |
| I | −861,085.82 | ||||||
| 6 | H | −836,749.36 | −8.07 | 2.20 |  | 0.037 |  |
| I | 836,728.71 | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grozav, A.; Porumb, I.-D.; Găină, L.I.; Filip, L.; Hanganu, D. Cytotoxicity and Antioxidant Potential of Novel 2-(2-((1H-indol-5yl)methylene)-hydrazinyl)-thiazole Derivatives. Molecules 2017, 22, 260. https://doi.org/10.3390/molecules22020260
Grozav A, Porumb I-D, Găină LI, Filip L, Hanganu D. Cytotoxicity and Antioxidant Potential of Novel 2-(2-((1H-indol-5yl)methylene)-hydrazinyl)-thiazole Derivatives. Molecules. 2017; 22(2):260. https://doi.org/10.3390/molecules22020260
Chicago/Turabian StyleGrozav, Adriana, Ioan-Dan Porumb, Luiza Ioana Găină, Lorena Filip, and Daniela Hanganu. 2017. "Cytotoxicity and Antioxidant Potential of Novel 2-(2-((1H-indol-5yl)methylene)-hydrazinyl)-thiazole Derivatives" Molecules 22, no. 2: 260. https://doi.org/10.3390/molecules22020260






