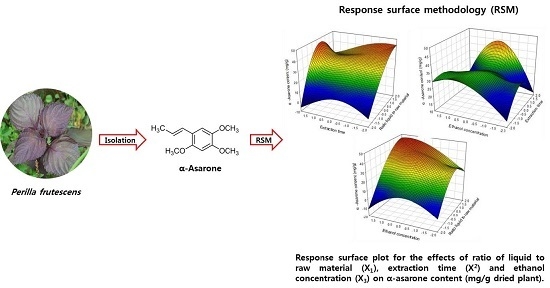
Rapid High Performance Liquid Chromatography Determination and Optimization of Extraction Parameters of the α-Asarone Isolated from Perilla frutescens L.
Abstract
:1. Introduction
2. Results and Discussion
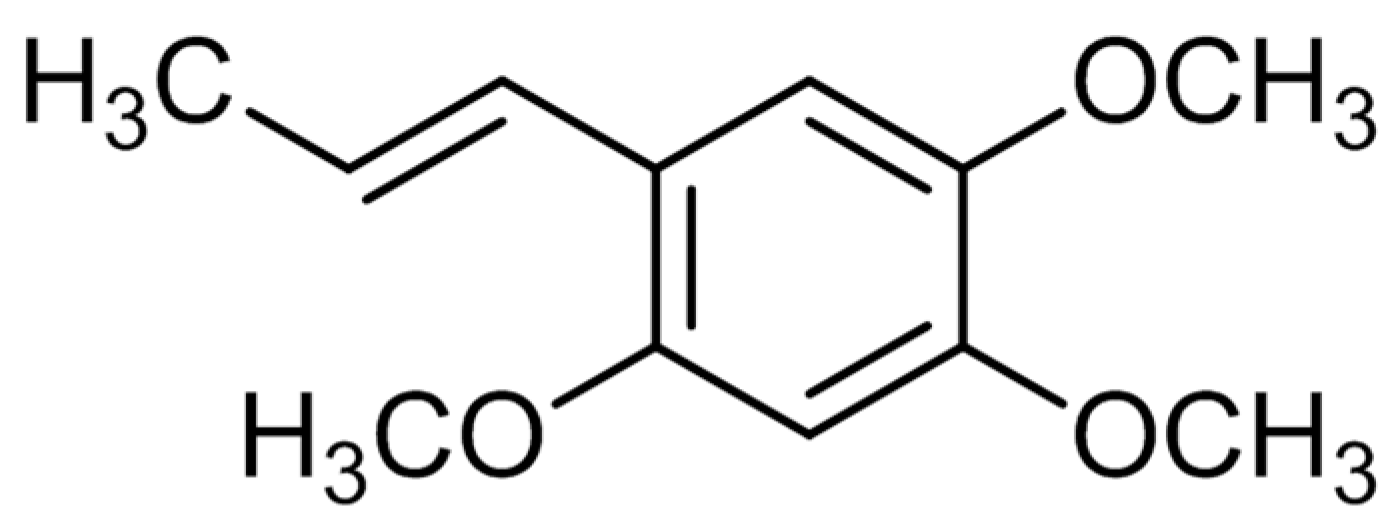
2.1. Structural Determination of Isolate Compounds
2.2. Linearity
2.3. Precision (Recovery)
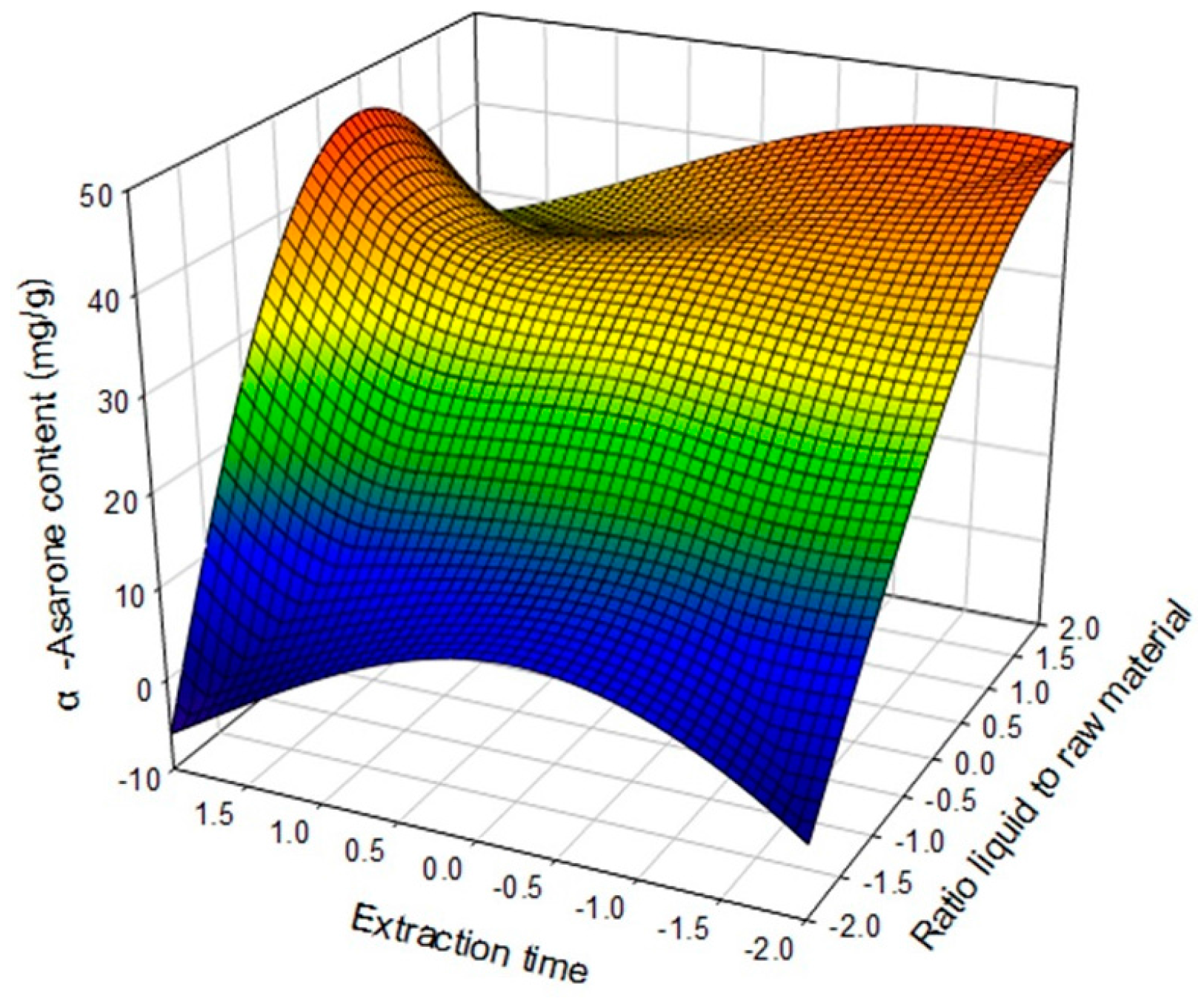
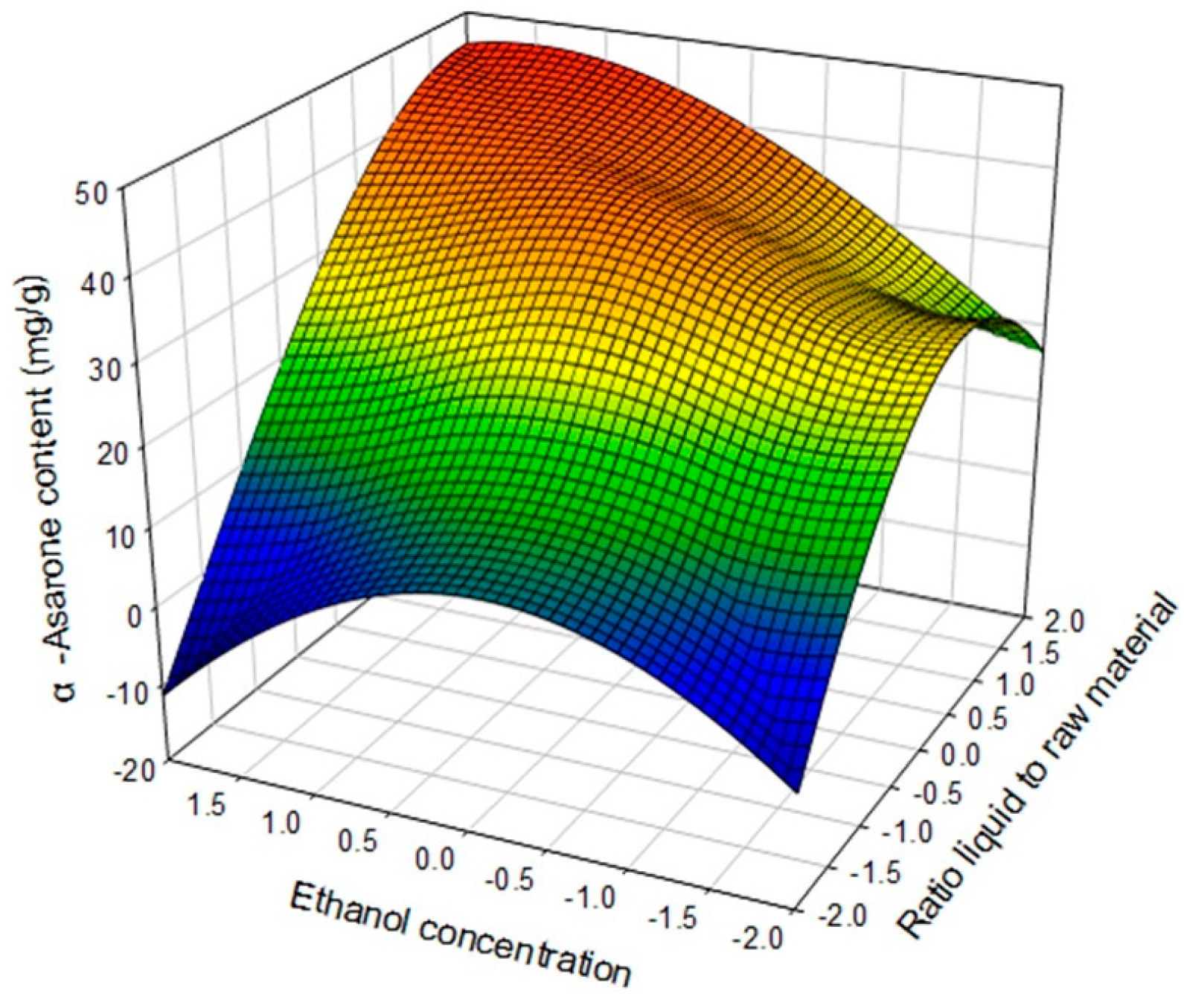
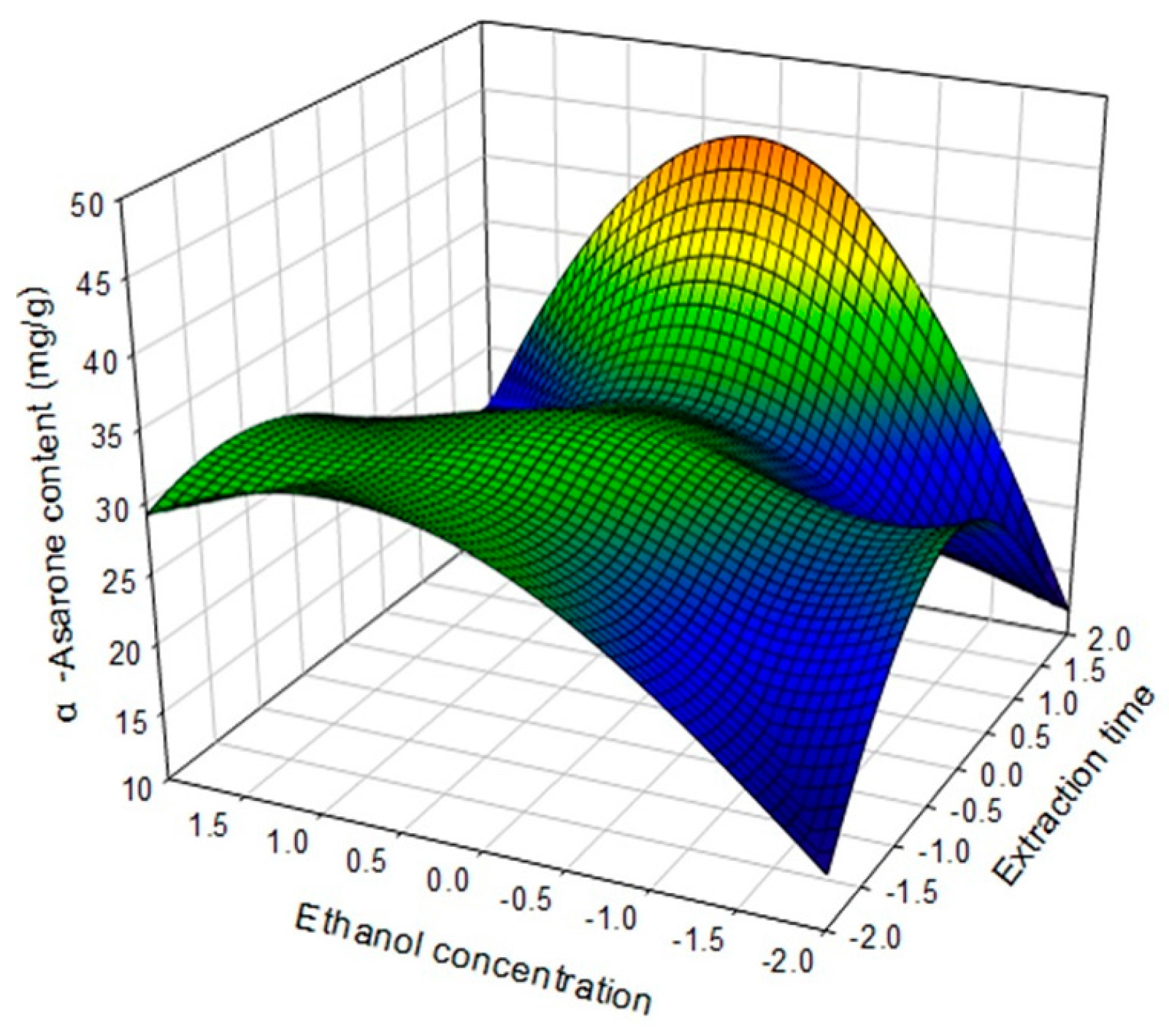
2.4. Optimization of Extraction Conditions
3. Materials and Methods
3.1. Plant Material, Extraction and Isolation
3.2. General Experimental Procedures
3.3. HPLC Analysis
3.4. Method Validation of α-Asarone from PF
3.5. Extraction Process
3.6. Experimental Design for RSM
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chiste, R.C.; Yamashita, F.; Gozzo, F.C.; Mercadante, A.Z. Simultaneous extraction and analysis by high performance liquid chromatography coupled to diode array and mass spectrometric detectors of bixin and phenolic compounds from annatto seeds. J. Chromatogr. A 2011, 1218, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Fry, O.; Česla, P.; Bajerová, P.; Adam, M.; Ventura, K. Optimization of focused ultrasonic extraction of propellant components determined by gas chromatography/mass spectrometry. Talanta 2012, 99, 316–322. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Shi, J.; Walid, E.; Zhang, H.; Ma, Y.; Xue, S.J. Reverse micellar extraction of lectin from black turtle bean (Phaseolus vulgaris): Optimisation of extraction conditions by response surface methodology. Food Chem. 2015, 166, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Aybastier, O.; Isik, E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013, 141, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Ghasemi, E.; Sasani, M. Application of response surface methodology for air assisted-dispersive liquid-liquid microextraction of deoxynivalenol in rice samples prior to HPLC-DAD analysis and comparison with solid phase extraction cleanup. Talanta 2017, 165, 27–32. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F. Prediction of the retention of s-triazines in reversed-phase high-performance liquid chromatography under linear gradient-elution conditions. J. Sep. Sci. 2014, 37, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F.; Carlucci, M.; Ferrone, V.; Carlucci, G. Optimisation by response surface methodology of microextraction by packed sorbent of non steroidal anti-inflammatory drugs and ultra-high performance liquid chromatography analysis of dialyzed samples. J. Pharm. Biomed. Anal. 2016, 125, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Aybastıer, Ö; Şahin, S.; Işık, E.; Demir, C. Determination of total phenolic content in Prunella L. by horseradish peroxidase immobilized onto chitosan beads. Anal. Methods 2011, 3, 2289–2297. [Google Scholar]
- Adham, A.N. Comparative extraction methods, phytochemical constituents, fluorescence analysis and HPLC validation of rosmarinic acid content in Mentha piperita, Mentha longifolia and Osimum basilicum. J. Pharmacogn. Phytochem. 2015, 3, 130–139. [Google Scholar]
- Zhao, M.; Ding, W.; Wang, S.W.; Wang, C.W.; Du, Y.; Xu, H.; Wang, Q.; Jin, S. Simultaneous determination of nine coumarins in rat plasma by HPLC–MS/MS for pharmacokinetics studies following oral administration of Fraxini Cortex extract. J. Chromatogr. B 2016, 1025, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Paek, J.H.; Lim, S.S. Simultaneous Ultra Performance Liquid Chromatography Determination and Antioxidant Activity of Linarin, Luteolin, Chlorogenic Acid and Apigenin in Different Parts of Compositae Species. Molecules 2016, 21, 1609. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.K.; Ham, H.M.; Jeong, M.H.; Kim, D.H.; Kim, H.J. Simultaneous determination of 15 phenolic compounds and caffeine in teas and mate using RP-HPLC/UV detection: Method development and optimization of extraction process. Food Chem. 2015, 172, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Oh, J. Validated HPLC method for the determination of fenofibric acid in rat plasma and its application to a comparative pharmacokinetic study of prodrugs JWU102 and fenofibrate. IJBMBS 2015, 2, 41–46. [Google Scholar]
- Liu, J.H.; Steigel, A.; Reininger, E.; Bauer, R. Two new prenylared 3-benzoxepin derivatives as cyclooxygenase inhibitors from Perilla frutescens var. acuta. J. Nat. Prod. 2000, 63, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Paek, J.H.; Shin, D.K.; Lee, J.Y.; Lim, S.S.; Kang, Y.H. Purple perilla extracts with α-asarone enhance cholesterol efflux from oxidized LDL-exposed macrophages. Int. J. Mol. Med. 2015, 35, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Vohora, S.B.; Shah, S.A.; Dandiya, P.C. Central nervous system studies on an ethanol extract of Acoruscalamus rhizomes. J. Ethnopharmacol. 1990, 28, 53–62. [Google Scholar] [CrossRef]
- Jianjia, C. Status epilepticus treated by α-asarone. Report of 18 cases. J. Tradit. Chin. Med. 1984, 4, 217–218. [Google Scholar]
- Rodriguez-Paez, L.; Juarez-Sanchez, M.; Antunez-Solis, J.; Baeza, I.; Wong, C. α-Asarone inhibits HMG-CoA reductase, lowers serum LDL-cholesterol levels and reduces biliary CSI in hypercholesterolemic rats. Phytomedicine 2003, 10, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.D.; Butle, S.R.; Patil, S.D.; Jagtap, P.K. Validated stability indicating RP-HPLC method for simultaneous determination and in vitro dissolution studies of thiocolchicoside and diclofenac potassium from tablet dosage form. Arab. J. Chem. 2015, 8, 119–128. [Google Scholar] [CrossRef]
- Dewani, A.P.; Dabhand, S.M.; Bakal, R.L.; Gadewar, C.K.; Chandewar, A.V.; Patra, S. Development and validation of a novel RP-HPLC method for simultaneous determination of paracetamol, phenylephrine hydrochloride, caffeine, cetirizine and nimesulide in tablet formulation. Arab. J. Chem. 2015, 8, 591–598. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Ambati, P.; Ayyanna, C. Optimizing medium constituents and fermentation conditions for citric acid production from palmyra jaggery using response surface method. World J. Microbiol. Biotechnol. 2001, 17, 331–335. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Zhang, Q.; Li, Y.F.; Dong, L.L.; Liu, S.L. Optimization of ultrasound extraction of Alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohydr. Polym. 2015, 119, 101–109. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F. Investigation by response surface methodology of extraction of caffeine, gallic acid and selected catechins from tea using water-ethanol mixtures. Food Anal. Methods 2016, 9, 2773–2779. [Google Scholar]
- Cacace, J.E.; Mazza, G. Extraction of anthocyanins and other phenolics from black currants with sulfured water. J. Agric. Food Chem. 2002, 50, 5939–5946. [Google Scholar] [CrossRef] [PubMed]
- Larkeche, O.; Zermane, A.; Menial, A.H.; Crampon, C.; Badens, E. Supercritical extraction of essential oil from Juniperus communis L. needles: Application of response surface methodology. J. Supercrit. Fluids 2015, 99, 8–14. [Google Scholar] [CrossRef]
- Wani, S.M.; Jan, N.; Wani, T.A.; Ahmad, M.; Masoodi, F.A.; Gani, A. Optimization of antioxidant activity and total polyphenols of dried apricot fruit extracts (Prunus armeniaca L.) using response surface methodology (RSM). J. Saudi Soc. Agric. Sci. 2015. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
| Compound | Slope | Intercept | R2 1 | LOD 2 (μg/mL) | LOQ 3 (μg/mL) |
|---|---|---|---|---|---|
| α-Asarone | 5995.91 | −116.94 | 0.9999 | 0.10 | 0.29 |
| Concentration (μg/mL) | Precision | Recovery (%, Mean ± RSD, n = 3) | |||
|---|---|---|---|---|---|
| Intra-Day (n = 3) | Inter-Day (n = 3) | ||||
| Rt 1 | Area 2 | Rt | Area | ||
| 200 | 0.02 | 0.36 | 0.05 | 2.16 | 97.7 ± 1.28 |
| 100 | 0.02 | 0.06 | 0.01 | 2.80 | 97.6 ± 2.26 |
| 50 | 0.02 | 0.44 | 0.02 | 1.64 | 99.2 ± 1.23 |
| No. | X1 1 | X2 2 | X3 3 | α-Asarone Content (mg/g Dried Plant) | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | −1 (5) | −1 (1.5) | −1 (70) | 17.47 j | 17.45 |
| 2 | −1 (5) | −1 (1.5) | 1 (90) | 14.57 k | 13.38 |
| 3 | −1 (5) | 1 (2.5) | −1 (70) | 19.36 i | 21.61 |
| 4 | −1 (5) | 1 (2.5) | 1 (90) | 14.74 k | 14.97 |
| 5 | 1 (13) | −1 (1.5) | −1 (70) | 30.93 f,g | 33.49 |
| 6 | 1 (13) | −1 (1.5) | 1 (90) | 46.36 a | 40.51 |
| 7 | 1 (13) | 1 (2.5) | −1 (70) | 31.77 f | 35.33 |
| 8 | 1 (13) | 1 (2.5) | 1 (90) | 36.80 d | 39.78 |
| 9 | −2 (3) | 0 (2) | 0 (80) | 7.51 l | 6.81 |
| 10 | 2 (15) | 0 (2) | 0 (80) | 39.71 c | 37.45 |
| 11 | 0 (10) | −2 (1) | 0 (80) | 31.71 f | 34.39 |
| 12 | 0 (10) | 2 (3) | 0 (80) | 40.82 b | 37.24 |
| 13 | 0 (10) | 0 (2) | −2 (50) | 27.16 h | 24.83 |
| 14 | 0 (10) | 0 (2) | 2 (100) | 30.73 g | 32.32 |
| 15 | 0 (10) | 0 (2) | 0 (80) | 31.07 f,g | 33.59 |
| 16 | 0 (10) | 0 (2) | 0 (80) | 36.37 d | 33.59 |
| 17 | 0 (10) | 0 (2) | 0 (80) | 34.31 e | 33.59 |
| Response Variable | Second Order Polynomial Equations | R2 | p-Value |
|---|---|---|---|
| α-Asarone (mg/g dried plant) | Yα-Asarone = 31.067841 + 13.243971X1 a + 2.368721X2 b + 2.013598X3 c − 9.165851X12 − 1.740172X1X2 + 2.234780X22 + 10.400973X1X3 + 3.200789X2X3 − 4.446175X32 | 0.9354 | 0.001 |
| Responses Variable | Optimum Extraction Conditions | Values | Morphology | |||
|---|---|---|---|---|---|---|
| X1 1 | X2 2 | X3 3 | Predict | Experimental | ||
| α-Asarone content (mg/g dried plant) | 10:13.5 | 1.76 | 90.37 | 40.56 b | 43.84 a | Saddle point |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.H.; Kwon, S.H.; Kang, Y.-H.; Lee, J.-Y.; Lim, S.S. Rapid High Performance Liquid Chromatography Determination and Optimization of Extraction Parameters of the α-Asarone Isolated from Perilla frutescens L. Molecules 2017, 22, 270. https://doi.org/10.3390/molecules22020270
Hwang SH, Kwon SH, Kang Y-H, Lee J-Y, Lim SS. Rapid High Performance Liquid Chromatography Determination and Optimization of Extraction Parameters of the α-Asarone Isolated from Perilla frutescens L. Molecules. 2017; 22(2):270. https://doi.org/10.3390/molecules22020270
Chicago/Turabian StyleHwang, Seung Hwan, Shin Hwa Kwon, Young-Hee Kang, Jae-Yong Lee, and Soon Sung Lim. 2017. "Rapid High Performance Liquid Chromatography Determination and Optimization of Extraction Parameters of the α-Asarone Isolated from Perilla frutescens L." Molecules 22, no. 2: 270. https://doi.org/10.3390/molecules22020270