Neoflavonoids as Inhibitors of HIV-1 Replication by Targeting the Tat and NF-κB Pathways
Abstract
:1. Introduction
2. Results and Discussion
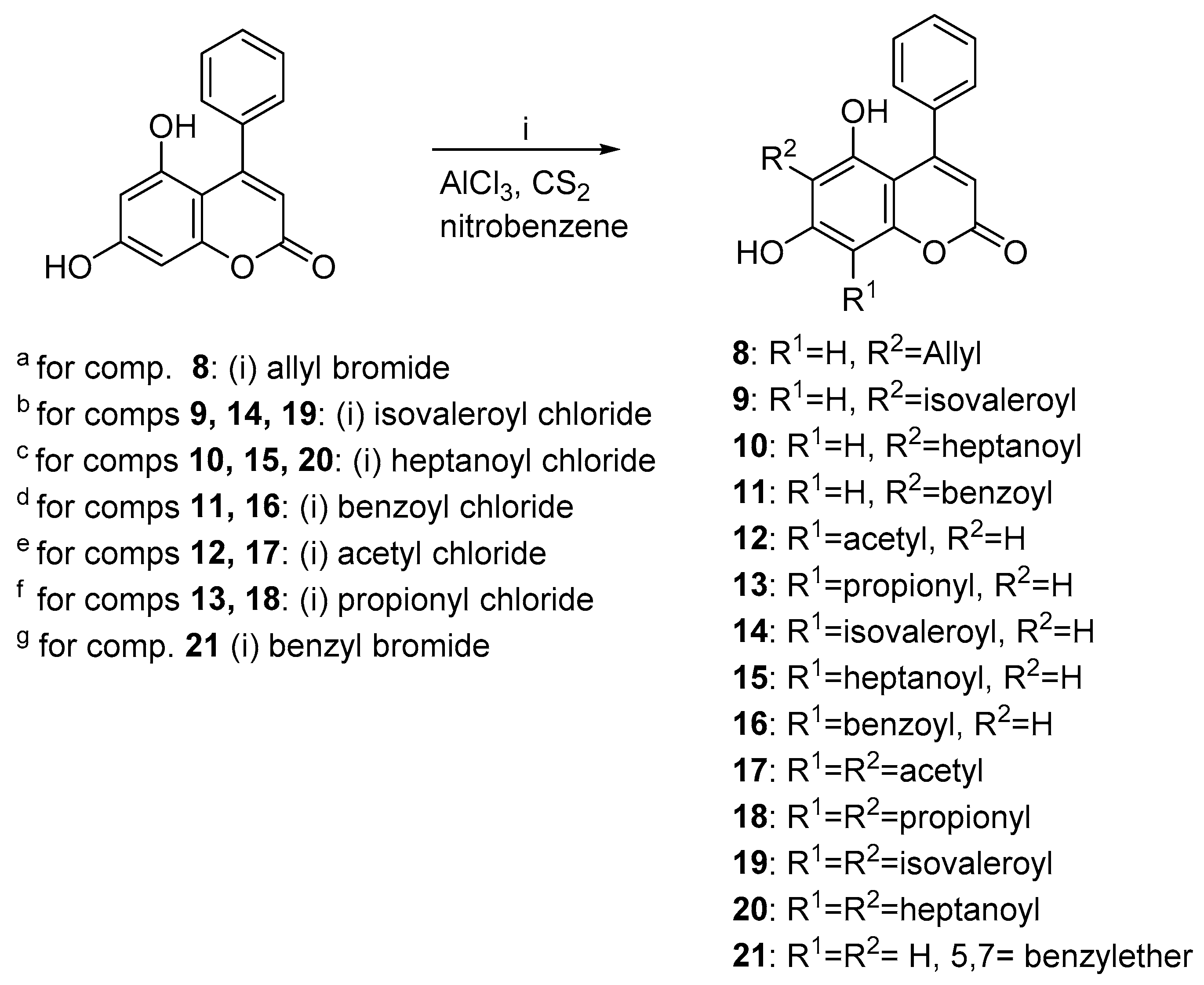
2.1. Chemistry
2.2. Evaluation of Antiviral Activity
3. Experimental Section
3.1. General Information
3.2. General Procedures I for the Synthesis of Compounds 1–7
3.3. General Procedures II: Synthesis of Compounds 8–22
3.4. General Procedures III: Synthesis of Compounds 22–28
3.5. Antiviral Activity Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- UNAIDS. The Joint United Nations Programme on HIV/AIDS. Report on the World Statistic the People Living with HIV. 2015. Available online: http://aidsinfo.unaids.org/ (accessed on 21 August 2016).
- Piot, P.; Bartos, M.; Ghys, P.D.; Walker, N.; Schwartlander, B. The global impact of HIV/AIDS. Nature 2001, 410, 968–973. [Google Scholar] [CrossRef]
- McNicholl, I.R.; McNicholl, J.J. On the horizon: Promising investigational antiretroviral agents. Curr. Pharm. Des. 2006, 12, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, M.A.; Jeang, K.T. 25 years of HIV-1 research—Progress and perspectives. BMC Med. 2008, 6. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. Challenges in the development of an HIV-1 vaccine. Nature 2008, 455, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Adamson, C.S.; Freed, E.O. Recent progress in antiretrovirals—Lessons from resistance. Drug Discov. Today 2008, 13, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Rabson, A.B.; Lin, H.C. NF-κB and HIV: Linking viral and immune activation. Adv. Pharmacol. 2000, 48, 161–207. [Google Scholar] [PubMed]
- Gatignol, A.; Duarte, M.; Daviet, L.; Chang, Y.N.; Jeang, K.T. Sequential steps in Tat trans-activation of HIV-1 mediated through cellular DNA, RNA, and protein binding factors. Gene Expr. 1996, 5, 217–228. [Google Scholar] [PubMed]
- Chen, B.K.; Feinberg, M.B.; Baltimore, D. The κB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J. Virol. 1997, 71, 5495–5504. [Google Scholar]
- Greene, W.C. The molecular biology of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 1991, 324, 308–317. [Google Scholar] [PubMed]
- Alcamí, J.; Lain de Lera, T.; Folgueira, L.; Pedraza, M.A.; Jacque, J.M.; Bachelerie, F.; Noriega, A.R.; Hay, R.T.; Harrich, D.; Gaynor, R.B. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995, 14, 1552–1560. [Google Scholar] [PubMed]
- Stevenson, M. Tat’s seductive side. Nat. Med. 2003, 9, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Yeni, P.G.; Hammer, S.M.; Carpenter, C.C.; Cooper, D.A.; Fischl, M.A.; Gatell, J.M.; Gazzard, B.G.; Hirsch, M.S.; Jacobsen, D.M.; Katzenstein, D.A.; et al. Antiretroviral treatment for adult HIV infection in 2002: Updated recommendations of the International AIDS Society-USA Panel. JAMA 2002, 288, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Yamamoto, Y.; Wang, Q.M. The IKK NF-κB system: A treasure trove for drug development. Nat. Rev. Drug Discov. 2004, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ferchichi, L.; Derbre, S.; Mahmood, K.; Toure, K.; Guilet, D.; Litaudon, M.; Awang, K.; Hadi, A.H.A.; Le Ray, A.M.; Richomme, P. Bioguided fractionation and isolation of natural inhibitors of advanced glycation end-products (AGEs) from Calophyllum flavoramulum. Phytochemistry 2012, 78, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenzan, M.A.; Nakamura, C.V.; Dias Filho, B.P.; Ueda-Nakamura, T.; Young, M.C.M.; García Cortez, D.A. Antileishmanial activity of crude extract and coumarin from Calophyllum brasiliense leaves against Leishmania amazonensis. Parasitol. Res. 2007, 101, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Guilet, D.; Helesbeux, J.-J.; Seraphin, D.; Sevenet, T.; Richomme, P.; Bruneton, J. Novel cytotoxic 4-phenylfuranocoumarins from Calophyllum dispar. J. Nat. Prod. 2001, 64, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilet, D.; Seraphin, D.; Rondeau, D.; Richomme, P.; Bruneton, J. Cytotoxic coumarins from Calophyllum dispar. Phytochemistry 2001, 58, 571–575. [Google Scholar] [CrossRef]
- Kashman, Y.; Gustafson, K.R.; Fuller, R.W.; Cardellina, J.H.; McMahon, J.B.; Currens, M.J.; Buckheit, R.W.; Hughes, S.H.; Cragg, G.M.; Boyd, M.R. HIV inhibitory natural products. Part 7. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J. Med. Chem. 1992, 35, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Games, D.E. Identification of 4-phenyl and 4-alkylcoumarins in Mammea americana L., Mammea africana G. Don and Calophyllum inophyllum by gas chromatography. Mass Spectrometry. Tetrahedron Lett. 1972, 13, 3187–3190. [Google Scholar] [CrossRef]
- Reutrakul, V.; Leewanich, P.; Tuchinda, P.; Pohmakotr, M.; Jaipetch, T.; Sophasan, S.; Santisuk, T. Cytotoxic coumarins from Mammea harmandii. Planta Med. 2003, 69, 1048–1051. [Google Scholar] [PubMed]
- Prachyawarakorn, V.; Mahidol, C.; Ruchirawat, S. NMR study of seven coumarins from Mammea siamensis. Pharm. Biol. 2000, 38, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Crombie, L.; Jones, R.C.F.; Palmer, C.J. Synthesis of the mammea coumarins. Part 1. The coumarins of the mammea A, B, and C series. J. Chem. Soc. Perkin Trans. 1 1987, 317–331. [Google Scholar] [CrossRef]
- Carpenter, I.; McGarry, E.J.; Scheinmann, F. Extractives from Guttiferae. Part XXI: The isolation and structure of nine coumarins from the bark of Mammea africana G. Don. J. Chem. Soc. C. 1971, 3783–3790. [Google Scholar] [CrossRef]
- Crombie, L.; Games, D.E.; McCormick, A. Extractives of Mammea americana L. Part II. The 4-phenylcoumarins isolation and structure of Mammea A/AA, A/A cyclo D, A/BA, A/AB, and A/BB. J. Chem. Soc. C. 1967, 2255–2260. [Google Scholar] [CrossRef]
- Rouger, C.; Derbré, S.; Charreau, B.; Pabois, A.; Cauchy, T.; Litaudon, M.; Awang, K.; Richomme, P. Lepidotol A from Mesua lepidota Inhibits Inflammatory and Immune Mediators in Human Endothelial Cells. J. Nat. Prod. 2015, 78, 2187–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng Lian, G.; Sin The, S.; Hui Mah, S.; Rahmani, M.; Taufiq-Yap, Y.H.; Awang, K. A Novel Cyclodione Coumarin from the Stem Bark of Mesua beccariana. Molecules 2011, 16, 7249–7255. [Google Scholar]
- Awang, K.; Chan, G.; Litaudon, M.; Ismail, N.H.; Martin, M.-T.; Gueritte, F.O. 4-Phenylcoumarins from Mesua elegans with acetylcholinesterase inhibitory activity. Bioorg. Med. Chem. 2010, 18, 7873–7877. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Lovaglio, E.; Vidari, G.; Finzi, P.V.; Neri, M.G.; Raimondi, A.; Parapini, S.; Taramelli, D.; Riva, A.; Bombardelli, E. 4-Alkyl- and 4-phenylcoumarins from Mesua ferrea as promising multidrug resistant antibacterials. Phytochemistry 2004, 65, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.; Dartiguelongue, C.; Youhana, T.; Oger, J.M.; Seraphin, D.; Duval, O.; Richomme, P.; Bruneton, J. New coumarins from Mesua racemosa: Isolation and synthesis. Heterocycles 1999, 51, 2183–2191. [Google Scholar]
- Morel, C.; Guilet, D.; Oger, J.M.; Seraphin, D.; Sevenet, T.; Wiart, C.; Hadi, A.H.A.; Richomme, P.; Bruneton, J. 6-Acylcoumarins from Mesua racemosa. Phytochemistry 1999, 50, 1243–1247. [Google Scholar] [CrossRef]
- Bala, K.R.; Seshadri, T.R. Isolation and synthesis of some coumarin components of Mesua ferrea seed oil. Phytochemistry 1971, 10, 1131–1134. [Google Scholar] [CrossRef]
- Cruz, F.G.; Moreira, L.d.M.; Santos, N.A.S.; Guedes, M.L.S. Additional Coumarins from Kielmeyera reticulate. J. Braz. Chem. Soc. 2002, 13, 704–707. [Google Scholar] [CrossRef]
- Cruz, F.G.; da Silva-Neto, J.T.; Guedes, M.L.S. Xanthones and Coumarins from Kielmeyera lathrophyton. J. Braz. Chem. Soc. 2001, 12, 117–122. [Google Scholar] [CrossRef]
- Gramacho, R.d.S.; Nagem, T.J.; de Oliveira, T.T.; de Queiroz, M.E.L.R.; Neves, A.A.; Saddi, N. Phenylcoumarins from Kielmeyera elata. Phytochemistry 1999, 51, 579–581. [Google Scholar] [CrossRef]
- Cruz, F.G.; Santos, N.A.S.; David, J.M.; Guedes, M.L.S.; Chávez, J.P. Coumarins from Kielmeyera argentea. Phytochemistry 1998, 48, 703–706. [Google Scholar] [CrossRef]
- Cruz, F.G.; Moreira, L.M.; David, J.M.; Guedes, M.L.S.; Chávez, J.P. Coumarins from Kielmeyera reticulata. Phytochemistry 1998, 47, 1363–1366. [Google Scholar]
- López-Pérez, J.L.; Olmedo, D.A.; del Olmo, E.; Vásquez, Y.; Solís, P.N.; Gupta, M.P.; San Feliciano, A. Cytotoxic 4-phenylcoumarins from the leaves of Marila pluricostata. J. Nat. Prod. 2005, 68, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. Chemistry of Anti HIV-1 Active Calophyllum Coumarins. J. Synth. Org. Chem. Jpn. 1998, 56, 116–124. [Google Scholar] [CrossRef]
- Chiang, C.C.; Mouscadet, J.F.; Tsai, H.J.; Liu, C.T.; Hsu, L.Y. Synthesis and HIV-1 integrase inhibition of novel bis- or tetra-coumarin analogues. Chem. Pharm. Bull. 2007, 55, 1740–1743. [Google Scholar] [CrossRef] [PubMed]
- Márquez, N.; Sancho, R.; Bedoya, L.M.; Alcamí, J.; López-Pérez, J.L.; San Feliciano, A.; Fiebich, B.L.; Muñoz, E. Mesuol, a natural occurring 4-phenylcoumarin, inhibits HIV-1 replication by targeting the NF-κB pathway. Antivir. Res. 2005, 66, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, L.M.; Beltrán, M.; Sancho, R.; Olmedo, D.A.; Sánchez-Palomino, S.; Olmo, E.; López-Pérez, J.L.; Muñoz, E.; San Feliciano, A.; Alcamí, J. 4-Phenylcoumarins as HIV transcription inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 4447–4450. [Google Scholar] [CrossRef] [PubMed]
- Krishna, C.; Bhargavi, M.V.; Rao, C.P.; Krupadanama, D. Synthesis and antimicrobial assessment of novel coumarins featuring 1,2,4-oxadiazole. Med. Chem. Res. 2015, 24, 3743–3751. [Google Scholar] [CrossRef]
- Chin, Y.P.; Huang, W.J.; Hsu, F.L.; Lin, Y.L.; Lin, M.H. Synthesis and evaluation of antibacterial activities of 5,7-Dihydroxycoumarin derivatives. Arch. Pharm. 2011, 344, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.H.; Jaki, B.U.; Klein, L.L.; Lankin, D.C.; McAlpine, J.B.; Napolitano, J.G.; Fryling, N.A.; Franzblau, S.G.; Cho, S.H.; Stamets, P.E.; et al. Chlorinated Coumarins from the Polypore Mushroom Fomitopsis officinalis and Their Activity against Mycobacterium tuberculosis. J. Nat. Prod. 2013, 76, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Huang, S.T.; Lee, F.W.; Kuo, H.S.; Lin, M.H. 6-Acyl-4-aryl/alkyl-5,7-dihydroxycoumarins as anti-inflammatory agents. Bioorg. Med. Chem. 2006, 14, 4402–4409. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, E. Final Report Project X.11. Iberoamerican Program of Science and Technology for Development: Madrid, Spain, 2004; (unpublished results). [Google Scholar]
- Cao, S.G.; Wu, X.H.; Sim, K.Y.; Tan, B.H.K.; Vittal, J.J.; Pereira, J.T.; Goh, S.H. Minor coumarins from Calophyllum teysmannii var. inophylloide and synthesis of cytotoxic calanone derivatives. Helv. Chim. Acta 1998, 81, 1404–1416. [Google Scholar] [CrossRef]
- Cao, S.G.; Sim, K.Y.; Goh, S.H. Three new coumarins from Calophyllum teysmannii var. inophylloide (Guttiferae). Heterocycles 1997, 45, 2045–2052. [Google Scholar]
- Kulkarni, M.V.; Kulkarni, G.M.; Lin, C.H.; Sun, C.M. Recent advances in coumarins and 1-azacoumarins as versatile biodynamic agents. Curr. Med. Chem. 2006, 13, 2795–2818. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.J.; Josephs, J.L. Synthesis of the Calophyllum coumarins. Part 2. J. Chem. Soc. Perkin Trans 1 1995, 3135–3152. [Google Scholar] [CrossRef]
- NAPROC-13 RMN Spectroscopic Database USAL. Available online: http://c13.usal.es (accessed on 17 February 2017).
- Gaurav Taneja, A.G.; Raghuvanshi, A.; Kant, R.; Maulik, P.R. Diversity-oriented general protocol for the synthesis of privileged oxygen scaffolds: Pyrones, coumarins, benzocoumarins and naphthocoumarins. Org. Biomol. Chem. 2013, 11, 5239–5253. [Google Scholar]
- Kamat, S.P.; D’Souza, A.M.; Paknikar, S.K.; Beauchamp, P.S. A convenient one-pot synthesis of 4-methyl-3-phenyl-, 3-aryl- and 3-aryl-4-phenylcoumarins. J. Chem. Res. Synop. 2002, 242–246. [Google Scholar] [CrossRef]
- Sancho, R.; Medarde, M.; Sánchez-Palomino, S.; Madrigal, B.M.; Alcamí, J.; Muñoz, E.; San Feliciano, A. Anti-HIV activity of some synthetic lignanolides and intermediates. Bioorg. Med. Chem. Lett. 2004, 14, 4483–4486. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from José Luis López-Pérez, E-Mail: [email protected].
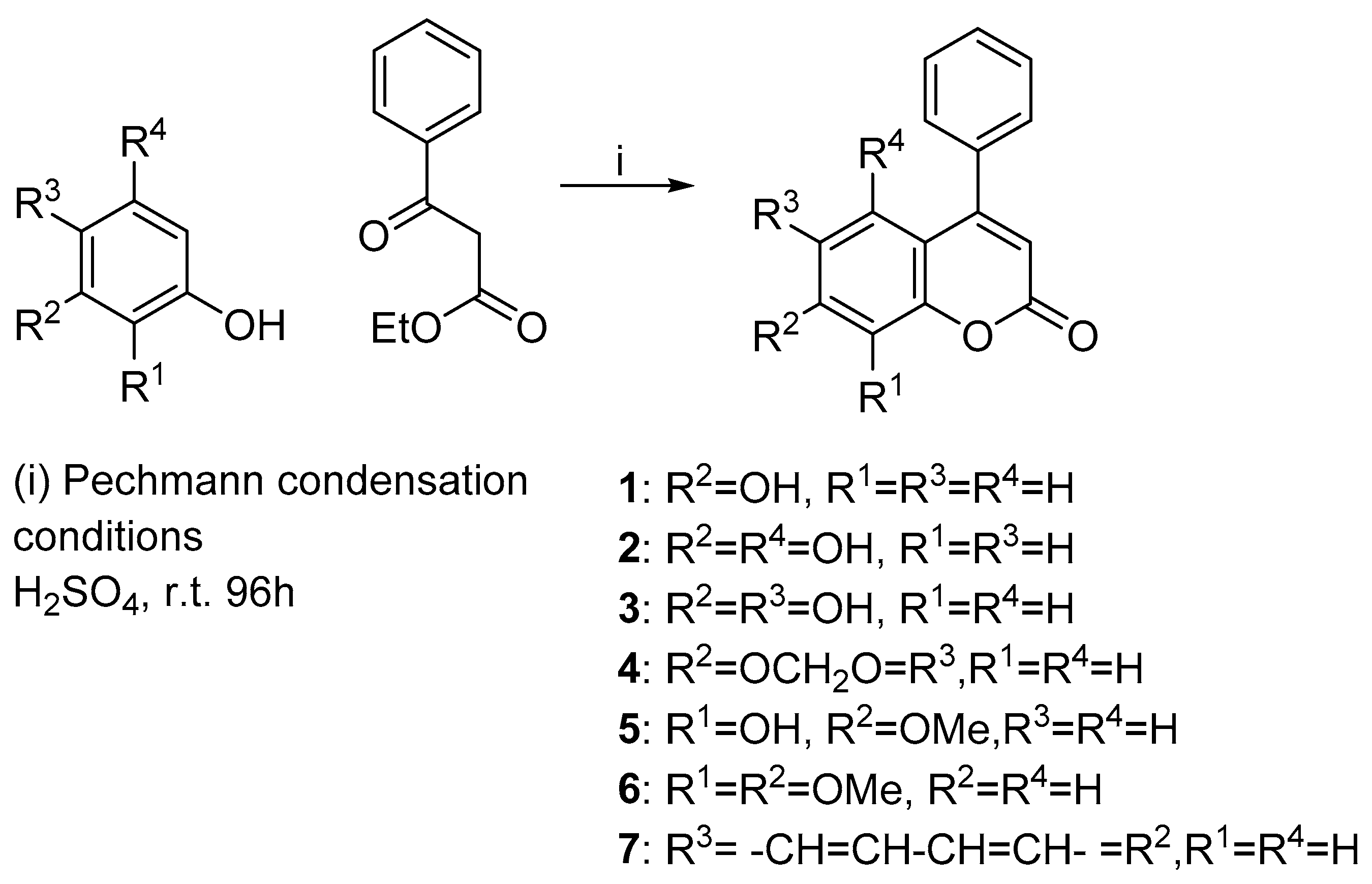

| Compound | NF-κB (5.1 LTR) | Hela-Tat-luc | Specificity (HeLa-Tet-On-Luc) | Toxicity MT2 (%) | ||
|---|---|---|---|---|---|---|
| 25 µM | 50 µM | 25 µM | 50 µM | 50 µM | 50 µM | |
| 1 | NT | −11.28 | NT | 27.88 | NT | 18.40 |
| 2 | NT | −4.10 | NT | −7.43 | NT | 8.78 |
| 3 | NT | 34.27 | NT | 6.59 | NT | 2.22 |
| 4 | 7.30 | 21.66 | 5.58 | 34.64 | S | 3.55 |
| 5 | 22.98 | 23.83 | −2.63 | 44.60 | S | 11.30 |
| 6 | NT | 19.12 | NT | 16.25 | NT | 2.88 |
| 7 | NT | 51.71 | NT | 94.69 | U | 4.00 |
| 8 | NT | 9.25 | NT | 21.74 | NT | 8.33 |
| 9 | NT | 68.74 | NT | 80.84 | U | NT |
| 10 | 70.53 | 68.19 | NT | 83.32 | S | <10 |
| 11 | NT | 37.81 | NT | 26.63 | S | NT |
| 12 | NT | 20.40 | NT | 5.81 | S | NT |
| 13 | NT | 67.29 | NT | 66.72 | U | NT |
| 14 | 83.06 | 86.60 | 41.87 | 69.32 | S | 17.02 |
| 15 | NT | 79.41 | NT | 80.37 | U | NT |
| 16 | NT | −17.05 | NT | 20.87 | S | NT |
| 17 | NT | 83.70 | NT | 44.93 | U | NT |
| 18 | NT | 35.05 | NT | 30.20 | S | NT |
| 19 | 59.86 | 66.04 | NT | 12.99 | S | NT |
| 20 | NT | 10.70 | NT | 22.30 | S | NT |
| 22 | NT | 15.20 | NT | 6.06 | NT | 1.61 |
| 23 | NT | 11.94 | NT | −34.54 | NT | 2.55 |
| 24 | 36.95 | 43.21 | NT | −18.03 | NT | 2.00 |
| 25 | 35.20 | 53.99 | 57.46 | 72.27 | S | 3.50 |
| 26 | NT | 13.37 | NT | −28.08 | NT | 2.59 |
| 27 | NT | 20.48 | NT | 15.08 | NT | 4.73 |
| 28 | NT | 5.67 | NT | −65.28 | NT | 6.13 |
| Mesuol | 71.00 | 77.90 | NT | 71.30 | S | >4 μM |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo, D.A.; López-Pérez, J.L.; Del Olmo, E.; Bedoya, L.M.; Sancho, R.; Alcamí, J.; Muñoz, E.; Feliciano, A.S.; Gupta, M.P. Neoflavonoids as Inhibitors of HIV-1 Replication by Targeting the Tat and NF-κB Pathways. Molecules 2017, 22, 321. https://doi.org/10.3390/molecules22020321
Olmedo DA, López-Pérez JL, Del Olmo E, Bedoya LM, Sancho R, Alcamí J, Muñoz E, Feliciano AS, Gupta MP. Neoflavonoids as Inhibitors of HIV-1 Replication by Targeting the Tat and NF-κB Pathways. Molecules. 2017; 22(2):321. https://doi.org/10.3390/molecules22020321
Chicago/Turabian StyleOlmedo, Dionisio A., José Luis López-Pérez, Esther Del Olmo, Luis M. Bedoya, Rocío Sancho, José Alcamí, Eduardo Muñoz, Arturo San Feliciano, and Mahabir P. Gupta. 2017. "Neoflavonoids as Inhibitors of HIV-1 Replication by Targeting the Tat and NF-κB Pathways" Molecules 22, no. 2: 321. https://doi.org/10.3390/molecules22020321






