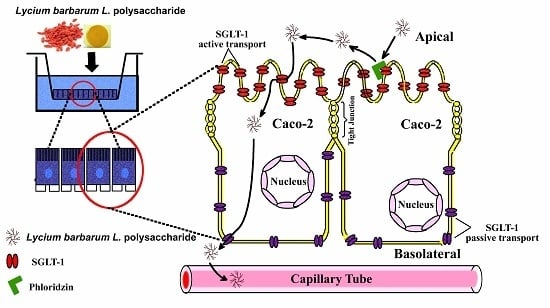
Lycium barbarum L. Polysaccharide (LBP) Reduces Glucose Uptake via Down-Regulation of SGLT-1 in Caco2 Cell
Abstract
:1. Introduction
2. Results
2.1. Characterization of LBP
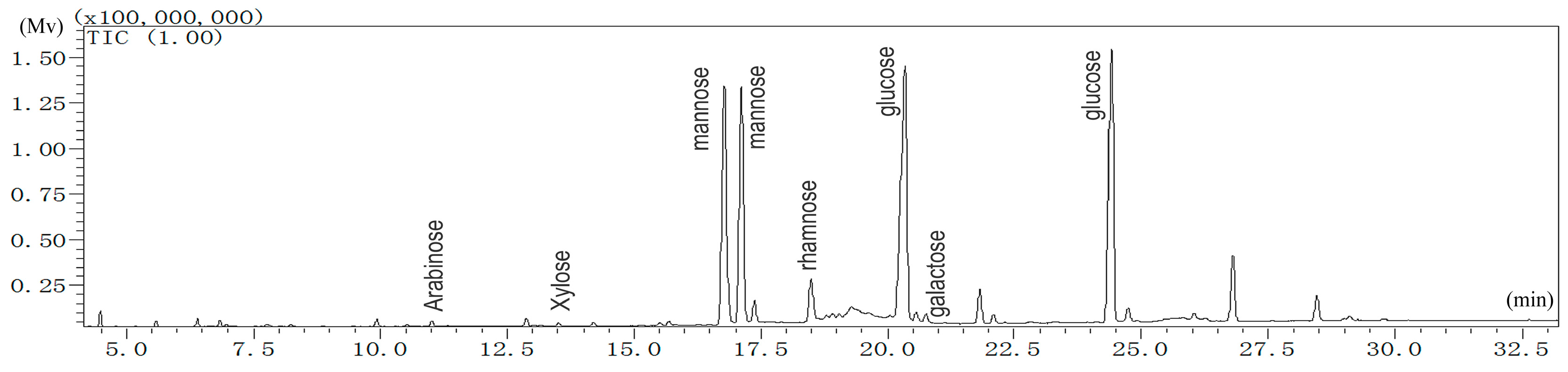
2.1.1. Monosaccharide Analysis of LBP
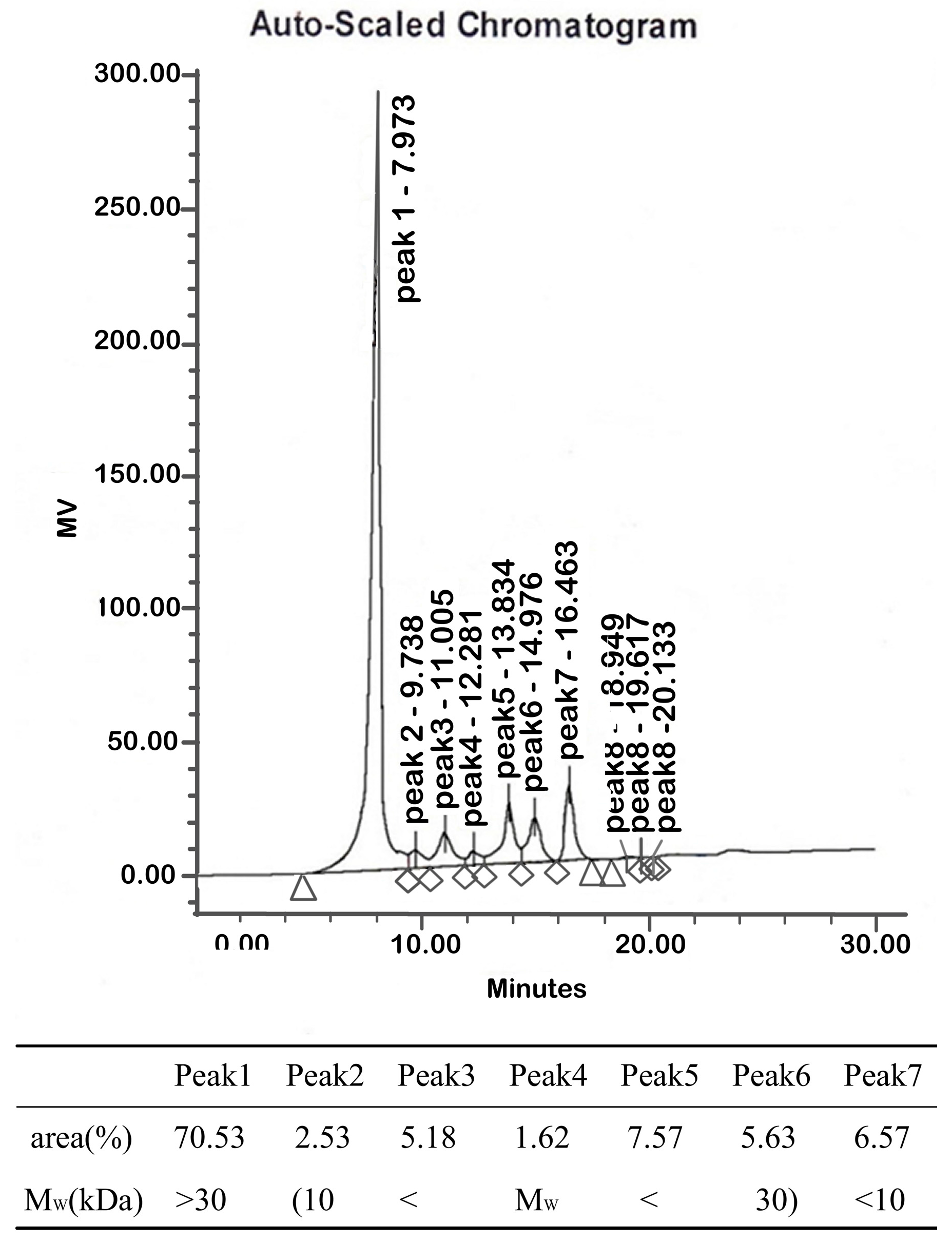
2.1.2. Molecular Weights
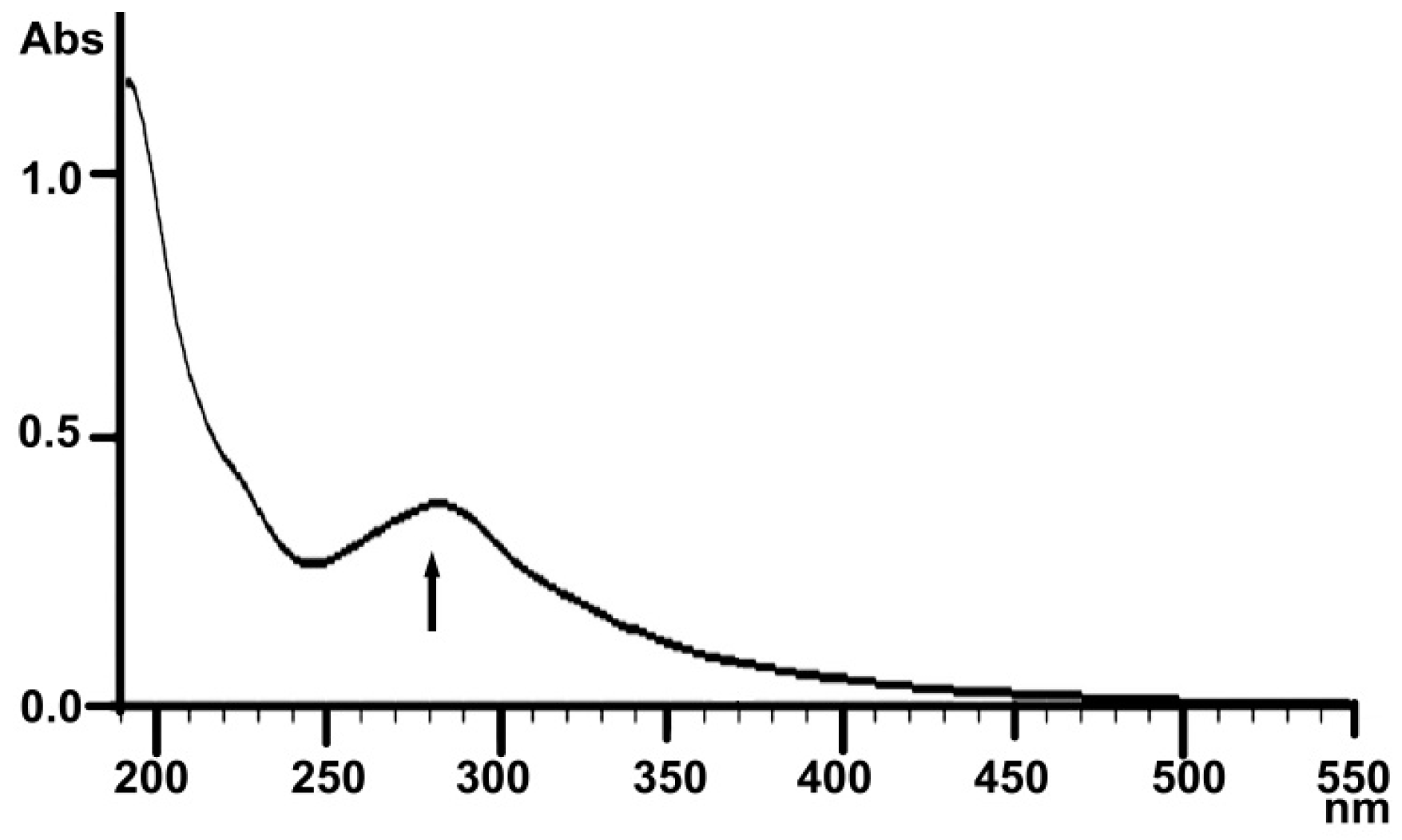
2.1.3. UV Analysis
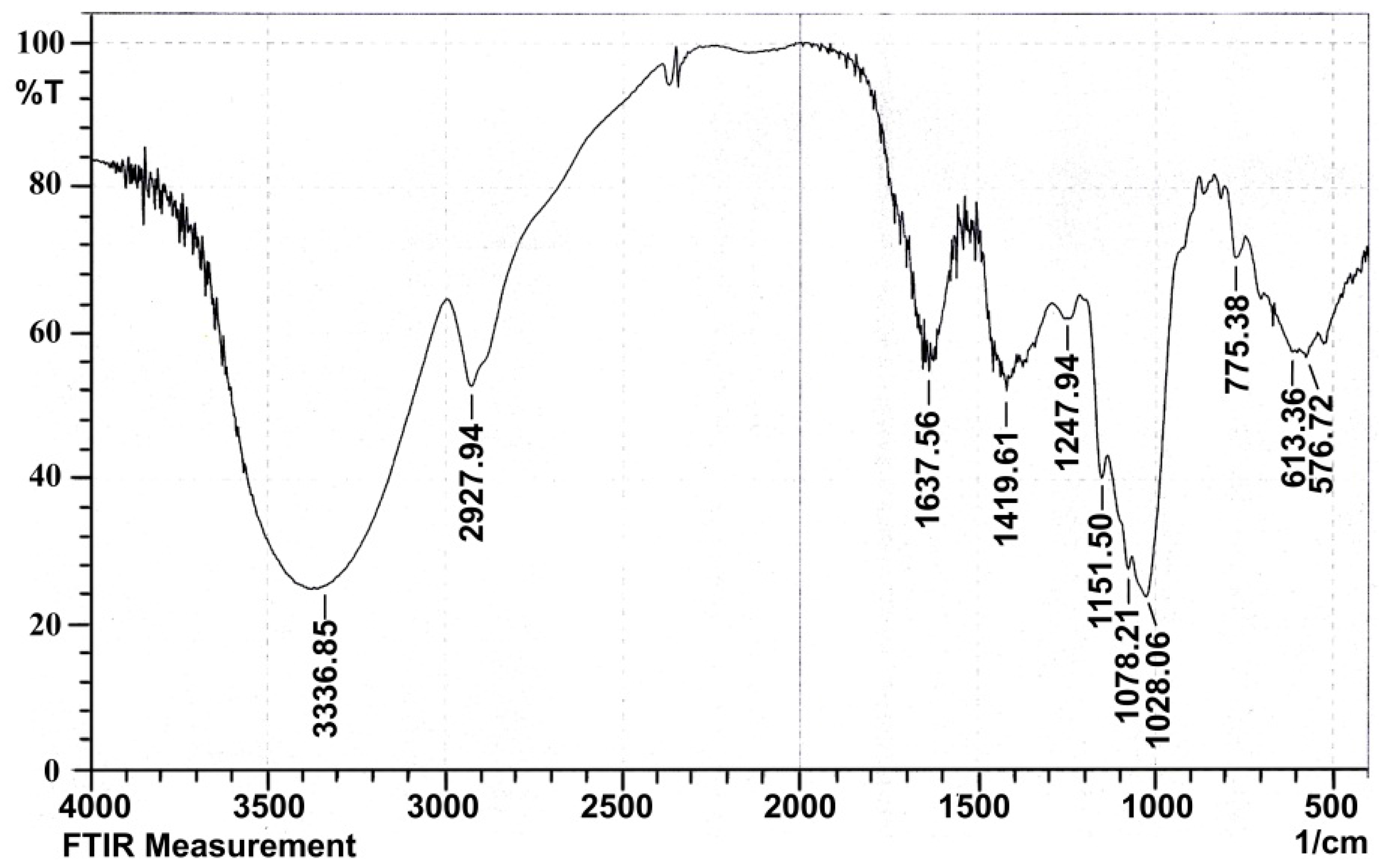
2.1.4. Infrared Spectral Analysis
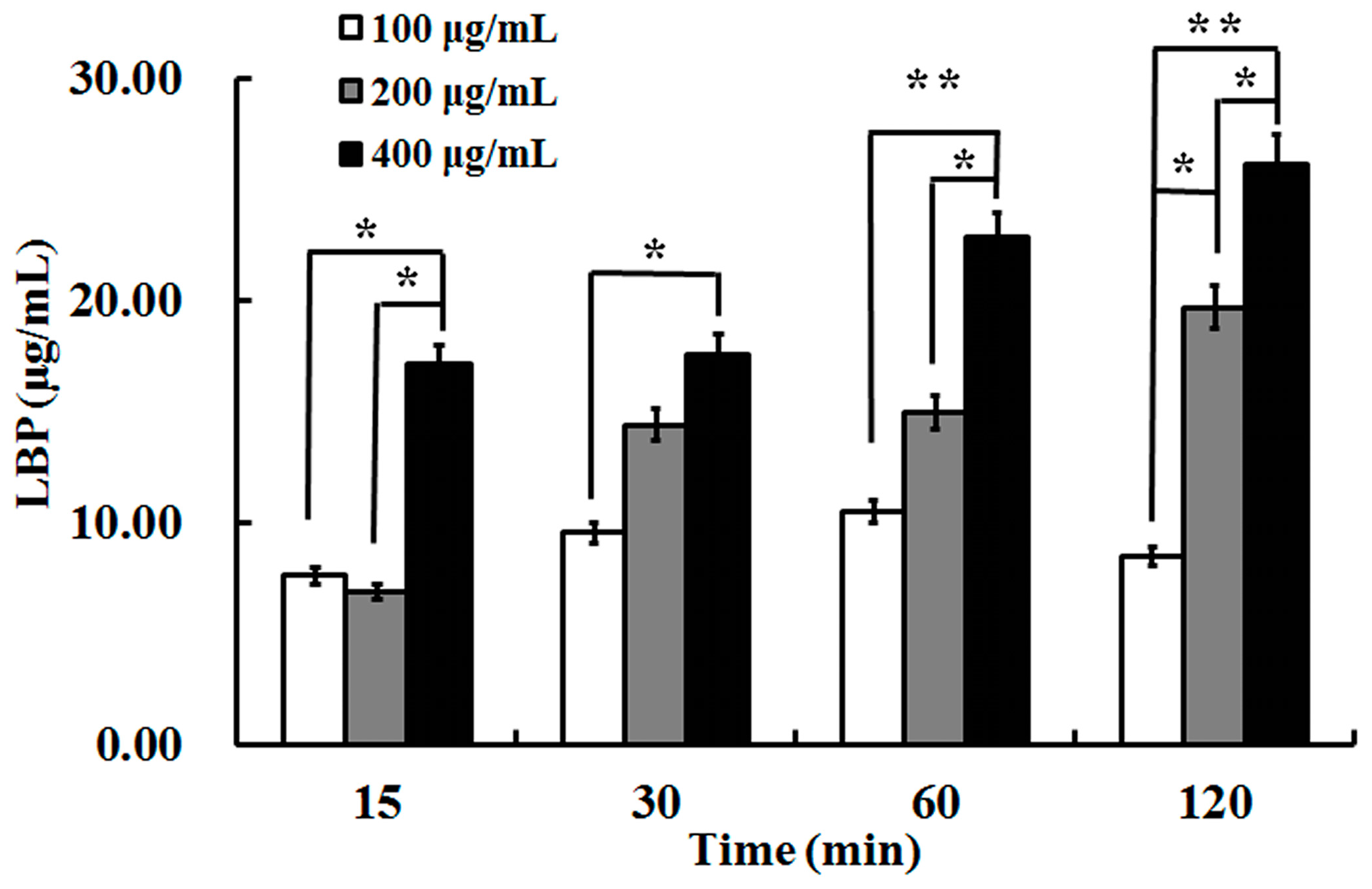
2.2. The Uptake of LBP by Caco2 Cell Monolayer
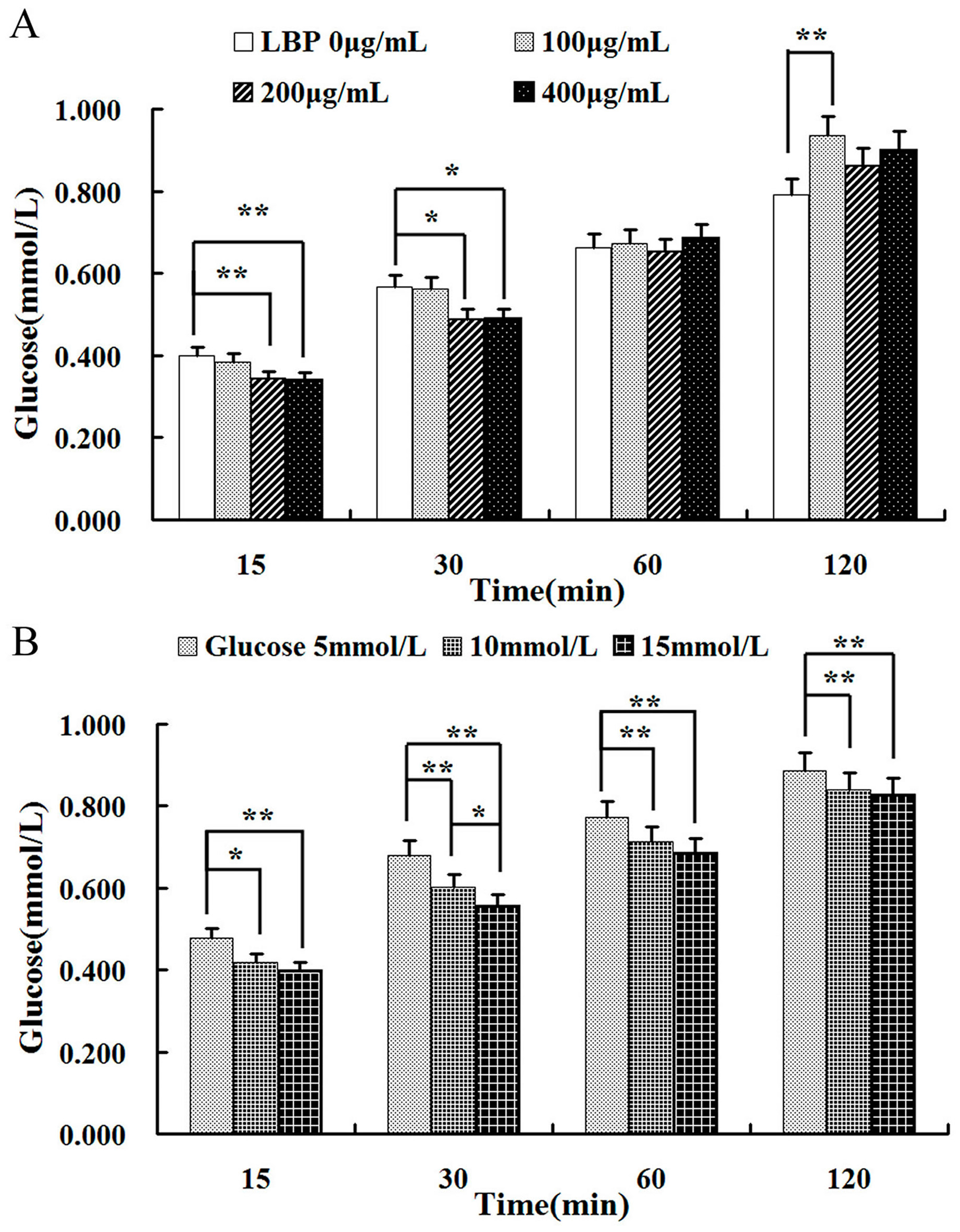
2.3. Effect of LBP on Glucose
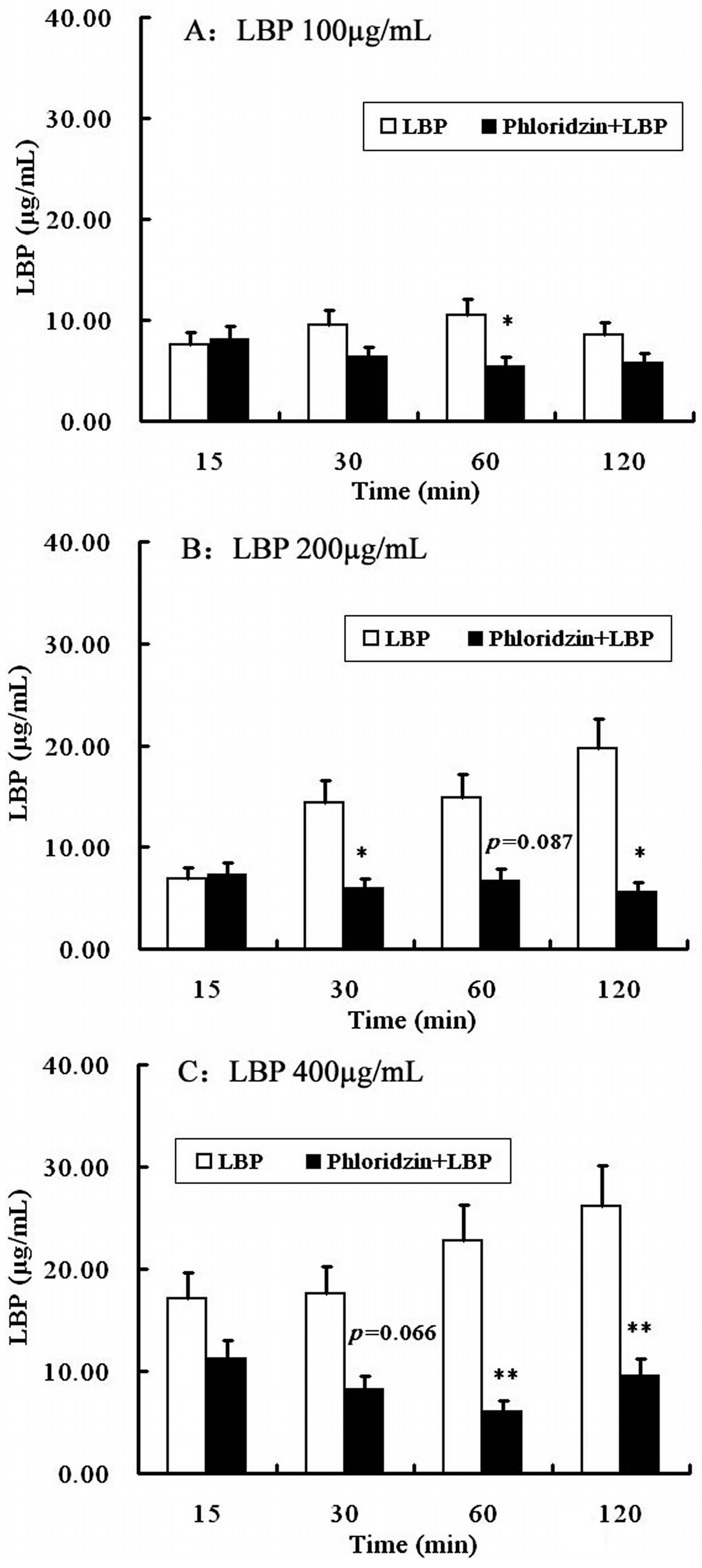
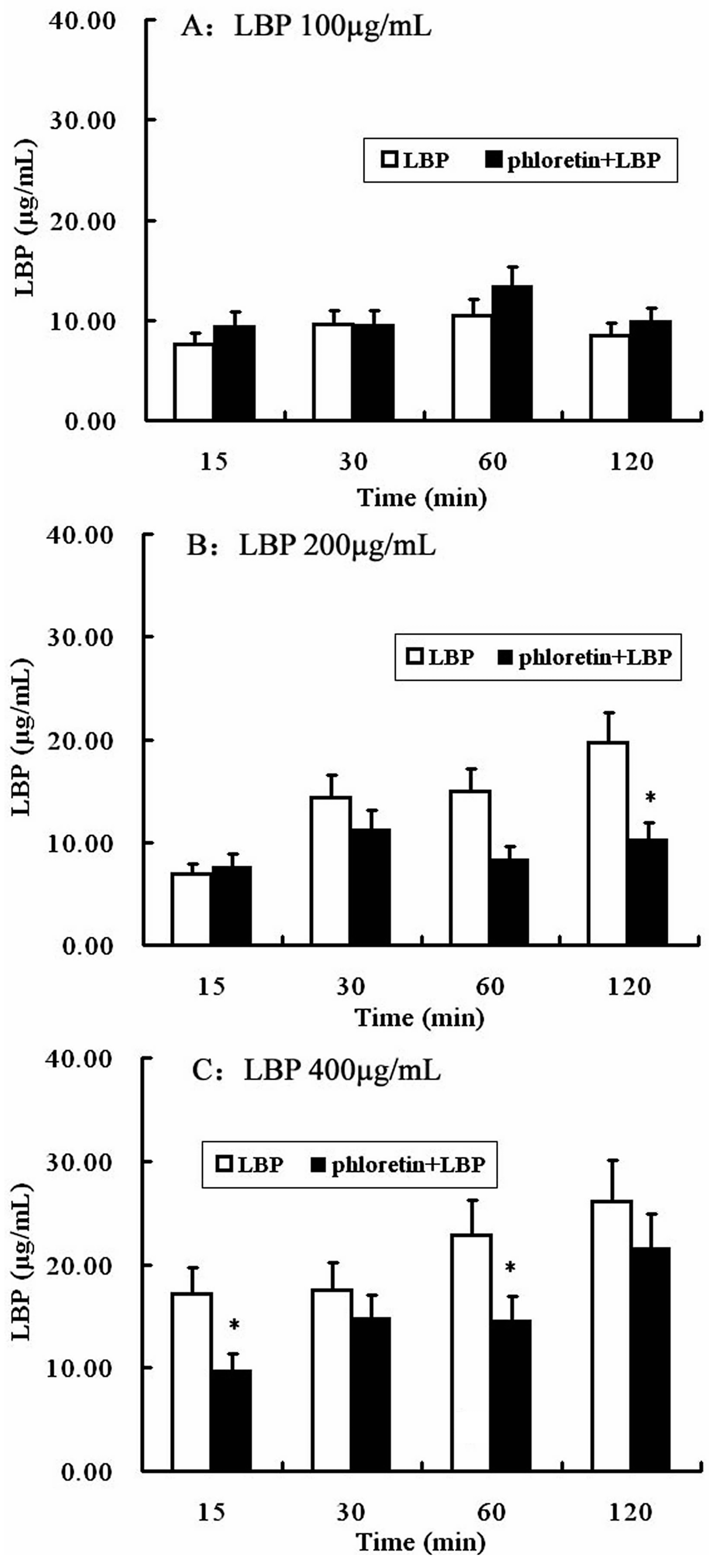
2.4. Involvement of SGLT-1 or GLUT-2 in the Uptake of LBP by Caco2 Monolayer
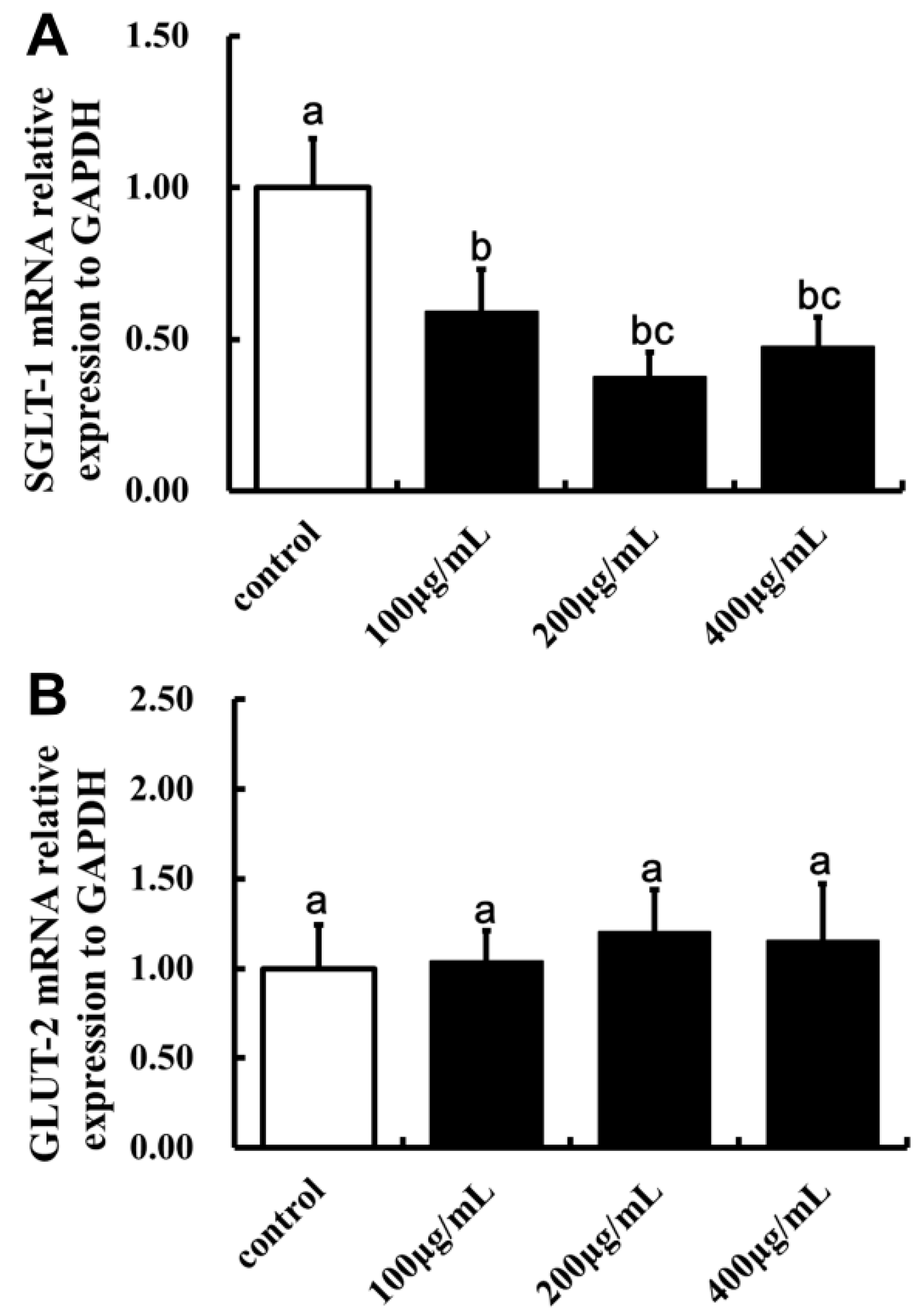
2.5. Effect of LBP on SGLT-1mRNA and GLUT-2mRNA Expression
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of LBP
4.3. Characterization of LBP
4.3.1. Analysis of Monosaccharide Composition
4.3.2. Molecular Weight Determination
4.3.3. UV and Infrared Spectral Analysis
4.4. Cell Culture and Cell Confluences
4.5. Uptake of LBP
4.6. Uptake of LBP on Glucose Consumption Assay
4.7. Effects of SGLT-1 or GLUT-2 during the Progress of LBP Uptake
4.7.1. SGLT-1 and GLUT-2 Inhibition Assay
4.7.2. SGLT-1mRNA and GLUT-2mRNA Expression
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, H.; Liu, F.; Zuo, P.; Huang, G.; Song, Z.; Wang, T.; Lu, H.; Guo, F.; Han, C.; Sun, G. Practical Application of Antidiabetic Efficacy of Lycium barbarum Polysaccharide in Patients with Type 2 Diabetes. Med. Chem. 2015, 11, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Heaton, K.W.; Marcus, S.N.; Emmett, P.M.; Bolton, C.H. Particle size of wheat, maize, and oat test meals: Effects on plasma glucose and insulin responses and on the rate of starch digestion in vitro. Am. J. Clin. Nutr. 1988, 47, 675–682. [Google Scholar] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Tang, H.L.; Chen, C.; Wang, S.K.; Sun, G.J. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int. J. Biol. Macromol. 2015, 77, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Kobayashi, M.; Tsukiyama, R.; Fujimoto, M.; Suzuki, M.; Tsuji, K.; Yamamoto, K. Stimulatory effect of Shoyu polysaccharides from soy sauce on the intestinal immune system. Int. J. Mol. Med. 2008, 22, 243–247. [Google Scholar]
- Garcia-Gonzalez, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [PubMed]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.R.; Rayment, P.; Wang, Q. A physico-chemical perspective of plant polysaccharides in relation to glucose absorption, insulin secretion and the entero-insular axis. Proc. Nutr. Soc. 1996, 55, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Nie, S.P.; Min, F.F.; Xie, M.Y. Artificial simulated saliva, gastric and intestinal digestion of polysaccharide from the seeds of Plantago asiatica L. Carbohydr. Polym. 2013, 92, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Wang, L.; Qian, H.; Qi, X.; Ding, X.; Hu, B.; Li, J. The effect of oat beta-glucan on in vitro glucose diffusion and glucose transport in rat small intestine. J. Sci. Food Agric. 2016, 96, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chaplin, M. Monosaccharides. In Carbohydrate Analysis—A Practical Approach, 2nd ed.; University of Oxford: Oxford, UK, 1986; p. 324. [Google Scholar]
- Kanamori, K.; Sano, K.; Nakao, M.; Nakagawa, K. The Coomassie brilliant blue G 250 method for protein quantitation. (2) The application of an automatic technique (author's transl.). Rinsho Byori. Jpn. J. Clin. Pathol. 1980, 28, 1153–1156. [Google Scholar]
- Honda, S.; Suzuki, S.; Kakehi, K.; Honda, A.; Takai, T. Analysis of the monosaccharide compositions of total non-dialyzable urinary glycoconjugates by the dithioacetal method. J. Chromatogr. 1981, 226, 341–350. [Google Scholar] [CrossRef]
- Briske-Anderson, M.J.; Finley, J.W.; Newman, S.M. The influence of culture time and passage number on the morphological and physiological development of Caco-2 cells. Proc. Soc. Exp. Biol. Med. 1997, 214, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Lycium barbarum L. are available from Bairuiyuan Gouqi co., Ltd.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Yang, X.; Cai, Q.; Ren, B.; Qiu, H.; Yao, Z. Lycium barbarum L. Polysaccharide (LBP) Reduces Glucose Uptake via Down-Regulation of SGLT-1 in Caco2 Cell. Molecules 2017, 22, 341. https://doi.org/10.3390/molecules22020341
Cai H, Yang X, Cai Q, Ren B, Qiu H, Yao Z. Lycium barbarum L. Polysaccharide (LBP) Reduces Glucose Uptake via Down-Regulation of SGLT-1 in Caco2 Cell. Molecules. 2017; 22(2):341. https://doi.org/10.3390/molecules22020341
Chicago/Turabian StyleCai, Huizhen, Xiaohui Yang, Qian Cai, Binbin Ren, Hongyan Qiu, and Zhiqing Yao. 2017. "Lycium barbarum L. Polysaccharide (LBP) Reduces Glucose Uptake via Down-Regulation of SGLT-1 in Caco2 Cell" Molecules 22, no. 2: 341. https://doi.org/10.3390/molecules22020341