3. Experimental Section
3.1. General Information
All manipulations were performed using Standard Schlenck techniques under Argon atmosphere. Chemicals were purchased from Sigma Aldrich and used without further purification. All solvents were purified and dried by MBraun SPS 800 solvent purification system. Column chromatography was performed using silica gel 60 (70–230 mesh). 1H-NMR and 13C-NMR spectra were recorded at 300 MHz and 75 MHz, respectively. Chemical shifts, δ, are reported in ppm relative to the internal standard TMS for both 1H- and 13C-NMR. The products were characterized by GC (gas chromatography). Quantitative GC analyses were performed with a GC-2010 Plus gas chromatography (SHIMADZU). The NMR studies were carried out in high-quality 5 mm NMR tubes. Signals are quoted in parts per million as δ downfield from tetramethylsilane (δ = 0.00) as an internal standard. NMR multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, m = multiplet signal. IR spectra were recorded on a 398 spectrophotometer (Perkin-Elmer, King Saud University, Ryadh, Saudi Arabia). MS spectra were recorded on a ((DART-TOF-MS) instrument at King Saud University, Ryadh, Saudi Arabia). Elemental microanalysis was performed on an ElementarVario El III Carlo Erba 1108 elemental analyzer (INRAP, Sidi Thabet, Tunisia) and the values found were within ±0.3% of the theoretical values. Melting points were determined with Kofler bench at Isste of Borj Cedria (Hammam Lif, University of Carthage, Borj Cedria, Tunisia).
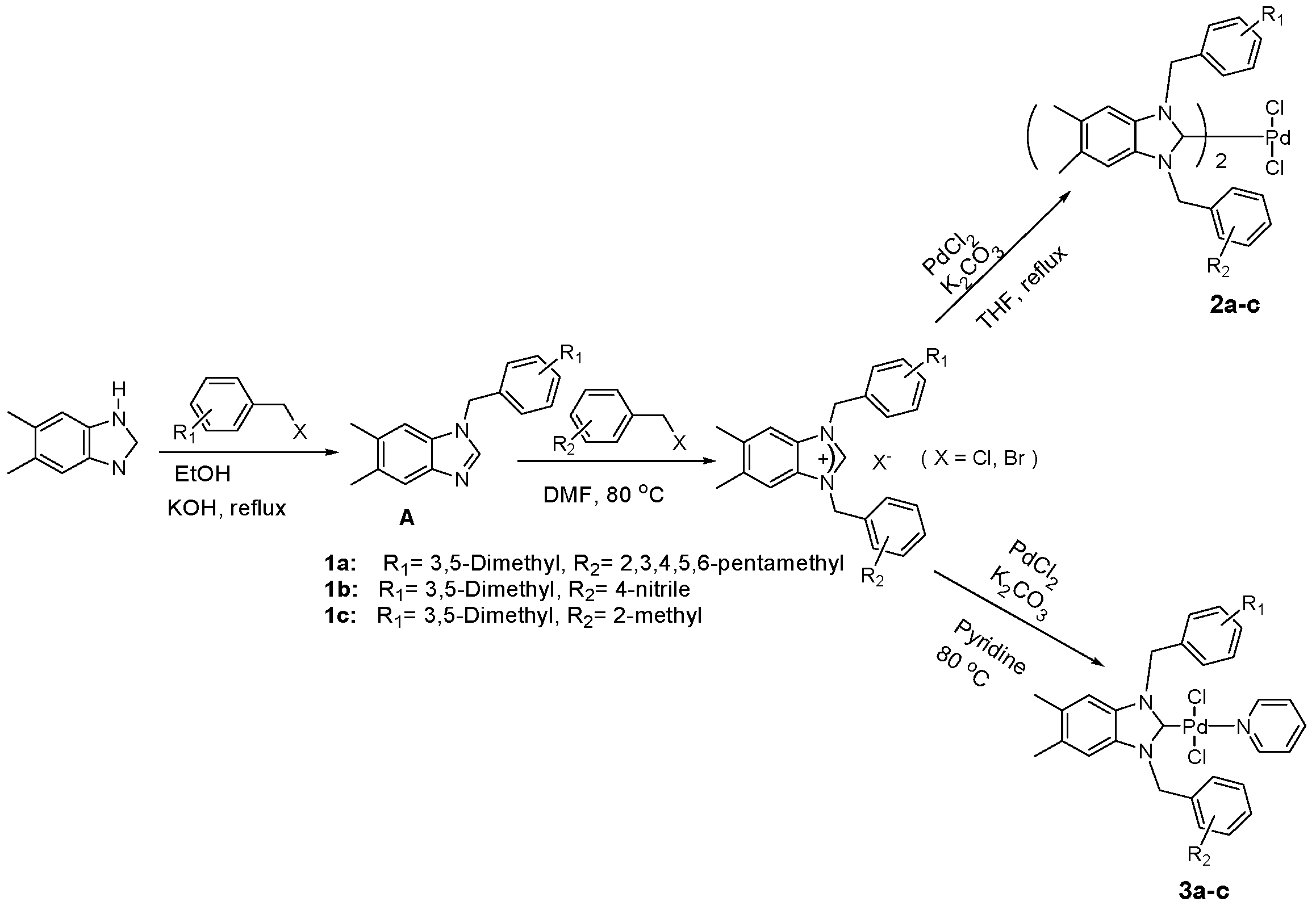
3.2. Synthesis of 1-(3,5-Dimethylbenzyl)-5,6-dimethylbenzimidazole (A)
To a solution of 5, 6-dimethylbenzimidazole (3 mmol, 4.38 g) resolved in 25 mL EtOH, (4 mmol, 2.5 g) of KOH was added and the reaction mixture was stirred for 15 min at room temperature. The corresponding aryl chlorides or bromides (3 mmol) were added slowly and the resulting mixture was stirred at room temperature for 1h and then heated for 8 h at 50 °C, after it was heated under reflux for 16 h. Solution was cooled to room temperature and the solvent was removed under reduced pressure. The yellow solid that formed was resolved with DCM (40 mL) and filtered. DCM was evaporated and the isolated product was characterized by NMR spectroscopy. Yield: 100(%). M.p. = 230 °C. FT-IR (KBr) ν, cm−1: 3065 (C-Harom); 1406 (C-N). 1H-NMR (CDCl3) δ (ppm):2.26 (s, 6H, Hc, d); 2.35 (s, 3H, Hb); 2.37 (s, 3H, Ha); 5.22 (s, 2H, H1′); 6.78 (s, 2H, H3′,7′); 6.93 (s, 1H, H5′); 7.08(s, 1H, H7); 7.58 (s, 1H, H4); 7.83 (s, 1H, H2).Anal. Calc. for C9H11N2: C, 73.437%; H, 7.532%; N, 19.031%, Found: C, 73.5; H, 7.6; N, 19.0%.
3.3. General Preparation of Benzimidazolium Salts 1a–c
To a solution of 5,6-dimethylbenzimidazole (3 mmol, 4.38 g) resolved in EtOH (25 mL) KOH (4 mmol, 2.5 g) was added and the reaction mixture was stirred for 15 min at room temperature. The corresponding aryl chlorides or bromides (3 mmol, 3equiv.) were added slowly and the resulting mixture was stirred at room temperature for 1h and then heated for 8 h at 50 °C, after it was heated under reflux for 16 h. Solution was cooled to room temperature and the solvent was removed under reduced pressure. The yellow solid that formed was resolved with DCM (40 mL) and filtered. DCM was evaporated and the isolated product was characterized by NMR spectroscopy.
A mixture of crude product (1 g) and corresponding aryl chlorides or bromides in DMF (2 mL) was stirred and heated at 70 °C for 1–2 days. The white solid that formed was washed with diethyl ether (30 mL), filtrated and dried under vacuum.
1-(3,5-Dimethylbenzyl)-5,6-dimethyl-3-(2,3,4,5,6-pentamethylbenzyl) benzimidazolium chloride (1a). Yield: 95 (%). M.p. = 225 °C. FT-IR (KBr) ν, cm−1: 3055 (C-Harom); 1545 (C-N); 1H-NMR (CDCl3) δ (ppm): 2.23 (s, 15H, He,f,g,h,i); 2.29 (s, 12H, Ha,b,c,d); 5.77 (s, 2H, H1′); 5.79 (s, 2H, H1′′); 6.87 (s, 2H, H3′,7′); 6.91 (s, 1H, H5′); 7.08 (s, 1H, H7); 7.24 (s, 1H, H4,); 10.91 (s, 1H, H2). 13C-NMR (CDCl3) δ (ppm): 16.65 (Cg); 16.80 (Cf,h); 16.99 (Ce,i); 20.49 (Cc,d); 20.90 (Ca,b); 47.47 (C1′′); 50.87 (C1′); 113 (C4,7); 124.84 (C5′); 125.01 (C4′,6′); 129.89 (C8,9); 130.14 (C4′′,6′′); 132.95 (C5′′); 133.27 (C5,6); 133.59 (C3′′,7′′); 136.74 (C2′); 136.88 (C2′′); 138.51 (C4′,6′); 141.73 (C2). Anal. Calc. for C30H38N2Cl: C, 77.977%; H, 8.289%; N, 6.062%, Found: C, 78.1; H, 8.3; N, 6.1%.
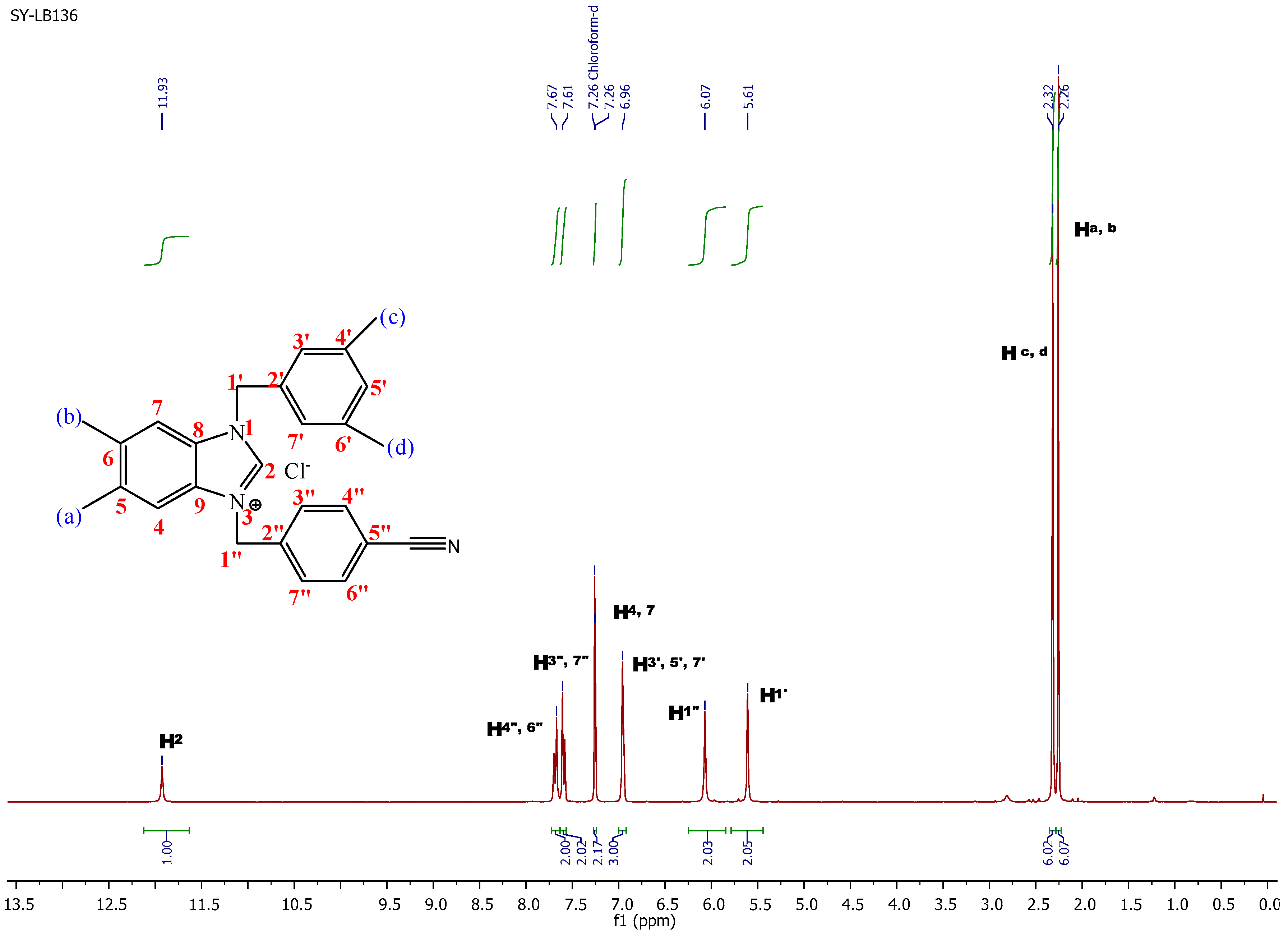
3-(4-Cyanobenzyl)-1-(3,5-dimethylbenzyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium chloride (1b). Yield: 90 (%). M.p. = 235 °C. FT-IR (KBr) ν, cm−1: 3062(C-Harom); 1570(C-N); 1H-NMR (CDCl3, δ (ppm): 2.26 (s, 6H, Ha,b); 2.32 (s, 6H, Hc,d); 5.61 (s, 2H, H1′); 6.07 (s, 2H, H1′′); 6.96 (s, 3H, H3′,5′,7′); 7.26 (s, 2H, H4,7); 7.61 (s, 2H, H3′′,7′′); 7.67 (s, 2H, H4′′,6′′); 11.93 (s, 1H, H2). 13C-NMR (CDCl3) δ (ppm): 20.81 (Ca,b); 21.34 (Cc,d); 50.46 (C1′′); 51.66 (C1′); 113.06 (C5′′); 113.58 (C4,7); 118.20 (CN); 125.64 (C5′); 129.10 (C3′,7′); 129.83 (C7′′); 129.93 (C3′′); 131.06 (C8,9); 132.42 (C4′′,6′′); 133.06 (C5,6); 137.74 (C6′); 137.82 (C4′); 138.52 (C2′); 139.28 (C2′′); 143.02 (C2). Anal. Calc. for C26H27N3Cl: C, 74.893%; H, 6.527%; N, 10.078%, Found: C, 74.9; H, 6.6; N, 10.1%.
1-(3,5-Dimethylbenzyl)-5,6-dimethyl-3-(2-methylbenzyl)benzo-1H-imidazol-3-ium chloride (1c). Yield: 79 (%). M.p. = 215 °C. FT-IR (KBr) ν, cm−1: 3064 (C-Harom); 1623(C-N); 1H-NMR (CDCl3) δ (ppm):2.26 (s, 9H, Hb,c,d); 2.32 (s, 3H, Ha); 2.40 (s, 3H, He); 5.72 (s, 2H, H1′); 5.85 (s, 2H, H1′′); 6.94 (s, 1H, H3′′); 7.00 (s, 2H, H3′,7′); 7.03 (s, 1H, H4′′); 7.09 (s, 1H, H5′′); 7.14 (s, 1H, H5′);7.22 (s, 1H, H7); 7.24 (s, 1H, H4); 7.28 (s, 1H, H6′′); 11.84 (s, 1H, H2). 13C-NMR (CDCl3) δ (ppm):19.63 (Ca,b); 20.79 (Ce); 21.33 (Cc, d); 50.02 (C1′′); 51.42 (C1′); 113.51 (C4, 7); 125.70 (C5′′); 126.77 (C5′); 127.88 (C3′,7′); 129.20 (C4′′); 130.02 (C3′′); 130.26 (C6′′); 130.86 (C9); 131.00 (C8); 131.36 (C5,6); 132.89 (C2′); 136.54 (C7′′); 137.35 (C2′′); 139.13 (C4′,6′); 143.39 (C2). Anal. Calc. for C26H30N2Cl: C, 76.919%; H, 7.448%; N, 6.900%, Found: C, 77.1; H, 7.5; N, 7.1%.
3.4. General Preparation of Palladium-bis-NHCs Complexes 2a–c
A Schlenk flask was charged with benzimidazolium salt (1 mmol), PdCl2 (0.5 mmol; 0.09 g), K2CO3 (0.6 g) and a stir bar under argon. Dried THF (25 mL) was then added as a solvent. The mixture was heated under reflux and stirred for 24 h at 100 °C. After completion, the reaction mixture was cooled at r.t. and the solvent was removed under vacuum. The solid formed was solubilized with DCM and purified by flash column, eluting with DCM until the product was completely recovered. DCM was removed under reduce pressure and the white solid was characterized by NMR spectroscopy. Further purification was done using recrystallization (DCM-hexane) or (DCM-CHCl3) to get pure complexes for analysis and catalysis.
Bis-[1-(3,5-dimethylbenzyl)-5,6-dimethyl-3-(2,3,4,5,6–pentamethylbenzyl)benzimidazoliN-2-ylidene] palladium (IV) dichloride (2a). Yield: 87 (%). M.p. = 233 °C. FT-IR (KBr) ν, cm−1: 3062 (C-Harom); 1445 (C-N); 1H-NMR (CDCl3) δ (ppm): 7.24 (s, 2H, H14, 14′, arom. CH); 7.07 (s, 2H, H12, 16, arom. CH); 6.92 (s, 1H, H12′, arom. CH); 6.83 (s, 1H, H16′, arom. CH); 6.73 (s, 2H, H4,7, arom. CH); 6.28 (s, 2H, H4′, 7′, arom. CH); 6.16 (s, 2H, H17, CH2); 6.02 (4H, H10, 10′, 2× CH2); 5.88 (2H, H17′, CH2); 2.42 (s, 6H, Hc, d, 2× CH3); 2.31 (s, 6H, Hc′, d′, 2× CH3); 2.28(s, 3H, Hg, CH3); 2.25 (s, 3H, Hg′, CH3); 2.22 (s, 12H, Ha, b, a′, b′, 4× CH3); 2.17 (s, 6H, He, i, 2× CH3); 2.15 (s, 12H, Hf,h,f′,h′, 4× CH3); 1.98 (s, 6H, He′,i′, 2× CH3). 13C-NMR (CDCl3) δ (ppm): 180.89 (NCN (C2,2′); 138.11–137.76 (arom. Cq (C8,9,8′,9′)); 136.09–135.90 (arom. Cq (C13,15,13′,15′); 135.34–135.12 (arom. Cq (C11,11′); 134.29 (arom. Cq (C18′,18); 133.16 (arom. Cq (C21,21′); 132.87–132.64 (arom. Cq (C20,22,20′,22′); 131.16 (arom. CH (C14,14′); 129.12–128.91 (arom. CH (C12,16,12′,16′); 125.41 (arom. Cq (C19,23,19′,23′); 125.18 (arom. Cq (C5,6,5′,6′); 112.37 (arom. CH (C4,7,4′,7′); 51.50 (CH2 (C10,10′); 50.86 (CH2 (C17,17′);16.82 (CH3 (Ce,i,e′,i′); 17.13 (CH3 (Cf,h,f′,h′); 17.57 (CH3 (Cg,g′); 20.46 (CH3 (Ca,b,a,b′); 21.05 (CH3 (Cc,d,c′,d′). (DART-TOF-MS) = (m/z = 732.32). Anal. Calc. for C60H74N4PdCl2: C, 70.062%; H, 7.252%; N, 5.447%, Found: C, 70.1; H, 7.3; N, 5.6%.
Bis-[3-(4-cyanolbenzyl)-1-(3,5-dimethylbenzyl)-5,6-dimethylbenzimidazoliN-2-ylidene] palladium (IV) dichloride (2b). Yield: 88 (%). M.p. = 234 °C. FT-IR (KBr) ν, cm−1: 3060 (C-Harom); 1462 (C-N); 1H-NMR (CDCl3) δ (ppm): 7.52 (s, 2H, H 4,7, arom. CH); 7.48 (s, 2H, H4′,7′, arom. CH); 7.41 (s, 4H, H19,23,19′,23′, arom. CH); 7.26 (s, 2H, H14,14′, arom. CH); 7.07 (s, 4H, H12,16,12′,16′, arom. CH); 6.95 (s, 2H, H20,22, arom. CH); 6.85 (s, 2H, H20′,22′, arom. CH); 5.97 (s, 2H, H10, CH2); 5.90 (s, 2H, H17, CH2); 5.82 (s, 2H, H17′, CH2); 5.76 (s, 2H, H10′, CH2); 2.24 (s, 12H, Hc,d,c′,d′, 4× CH3); 2.21–2.20 (s, 12H, Ha, b, a′, b′, 4× CH3). 13C-NMR (CDCl3) δ (ppm): 181.34 (NCN (C2,2′); 141.95 (arom. Cq (C8,9,8′,9′); 138.80 (arom. Cq (C18,18′); 136.06 (arom. Cq (C13,15,13′,15′); 133.47 (arom. Cq (C11,11′); 133.19 (arom. CH (C20,22); 133.00 (arom. CH (C20′,22′); 132.02 (arom. CH (C14,14′); 130.03–129.89 (arom. CH (C19,23,19′,23′); 128.44 (arom. CH (C12,16,12′,16′); 125.78–125.47 (arom. Cq (C5,6,5′,6′); 119.21 (CN); 112.21 (arom. CH (C4,7,4′,7′); 111.90 (arom. Cq (C21,21′); 52.08 (CH2 (C10,10′); 51.46 (CH2 (C17,17′); 21.76 (CH3 (Cc,d,c′,d′); 20.83 (CH3 (Ca,b,a′,b′). (DART-TOF-MS) (m/z = 642.32). Anal. Calc. for C52H52N6PdCl2: C, 66.560%; H, 5.586%; N, 8.956%, Found: C, 66.6; H, 5.6; N, 8.9%.
Bis-[1-(3,5-dimethylbenzyl)-5,6-dimethyl-3-(2-methylbenzyl)benzimidazoliN-2-ylidene] palladium (IV) dichloride (2c). Yield: 95 (%). M.p. = 245 °C. FT-IR (KBr) ν, cm−1: 3064 (C-Harom); 1463 (C-N); 1H-NMR (CDCl3) δ (ppm): 7.14 (s, 4H, H22,23,22′,23′, arom. CH); 7.09 (s, 4H, H14,14′,21,21′, arom. CH); 7.05 (s, 4H, H12,16,12′,16′, arom. CH); 6.94 (s, 1H, H4, arom. CH); 6.90 (s, 1H, H7, arom. CH); 6.84 (s, 1H, H4′, arom. CH); 6.81 (s, 1H, H7′, arom. CH); 6.73 (s, 1H, H20, arom. CH); 6.71 (s, 1H, H20′, arom. CH); 5.90 (s, 2H, H10, CH2); 5.84 (s, 2H, H10′, CH2); 5.75 (s, 2H, H17, CH2); 5.72 (s, 2H, H17′, CH2); 2.22 (s, 6H, Hc, d, 2× CH3); 2.21(s, 6H, Hc′,d′, 2× CH3); 2.18 (s, 12H, Ha,b,a′,b′, 4× CH3); 2.15 (s, 6H, He,e′, 4× CH3). 13C-NMR (CDCl3) δ (ppm): 181.91 (NCN (C2,2′); 138.55 (arom. Cq (C8,9,8′,9′); 136.50 (arom. Cq (C13,15,13′,15′); 135.63 (arom. Cq (C11,11′); 135.48 (arom. Cq (C19′,19); 134.69–134.51 (arom. Cq (C18,18′); 133.85–133.77 (arom. CH (C20,20′); 133.61–133.50 (arom. CH (C14,14′); 132.45 (arom. CH (C21,21′); 130.41 (arom. CH (C12,16); 129.71 (arom. CH (C12′,16′); 127.90–127.67 (arom. CH (C23,23′); 126.86 (arom. Cq (C5,6); 126.76 (arom. Cq (C5′,6′); 125.98–125.73 (arom. CH (C22,22′); 111.99–111.63 (arom. CH (C4,7,4′,7′); 52.20 (CH2 (C10,10′); 49.96 (CH2 (C17,17′); 21.70 (CH3 (Cc,d,c′,d′); 20.37 (CH3 (Ce,e′); 19.79 (CH3 (Ca,b,a′,b′). (DART-TOF-MS) (m/z = 383.2, m/z = 367.29). Anal. Calc. for C52H58N9PdCl2: C, 63.317%; H, 5.927%; N, 12.780%, Found: C, 63.4; H, 5.9; N, 12.8%.
3.5. General Preparation of PEPPSI Complexes 3a–c
A pressure tube was charged with benzimidazolium salts (1 mmol), PdCl2 (1 mmol; 0.18 g), K2CO3 (0.6 g) and a stir bar under atmosphere. Pyridine (1 mmol, 3 mL) was then added as the solvent and the reactant. The mixture was heated and stirred for 16 h at 80 °C. After cooling to r.t, the reaction mixture was diluted with CH2Cl2 and purified by flash column, eluting with DCM until the product was completely recovered. DCM was evaporated and the crude product was washed with 3 × 20 mL hexane. The yellow solid was characterized by NMR spectroscopy. Further purification was done using recrystallization (DCM-hexane) to get pure complexes for analysis and catalysis.
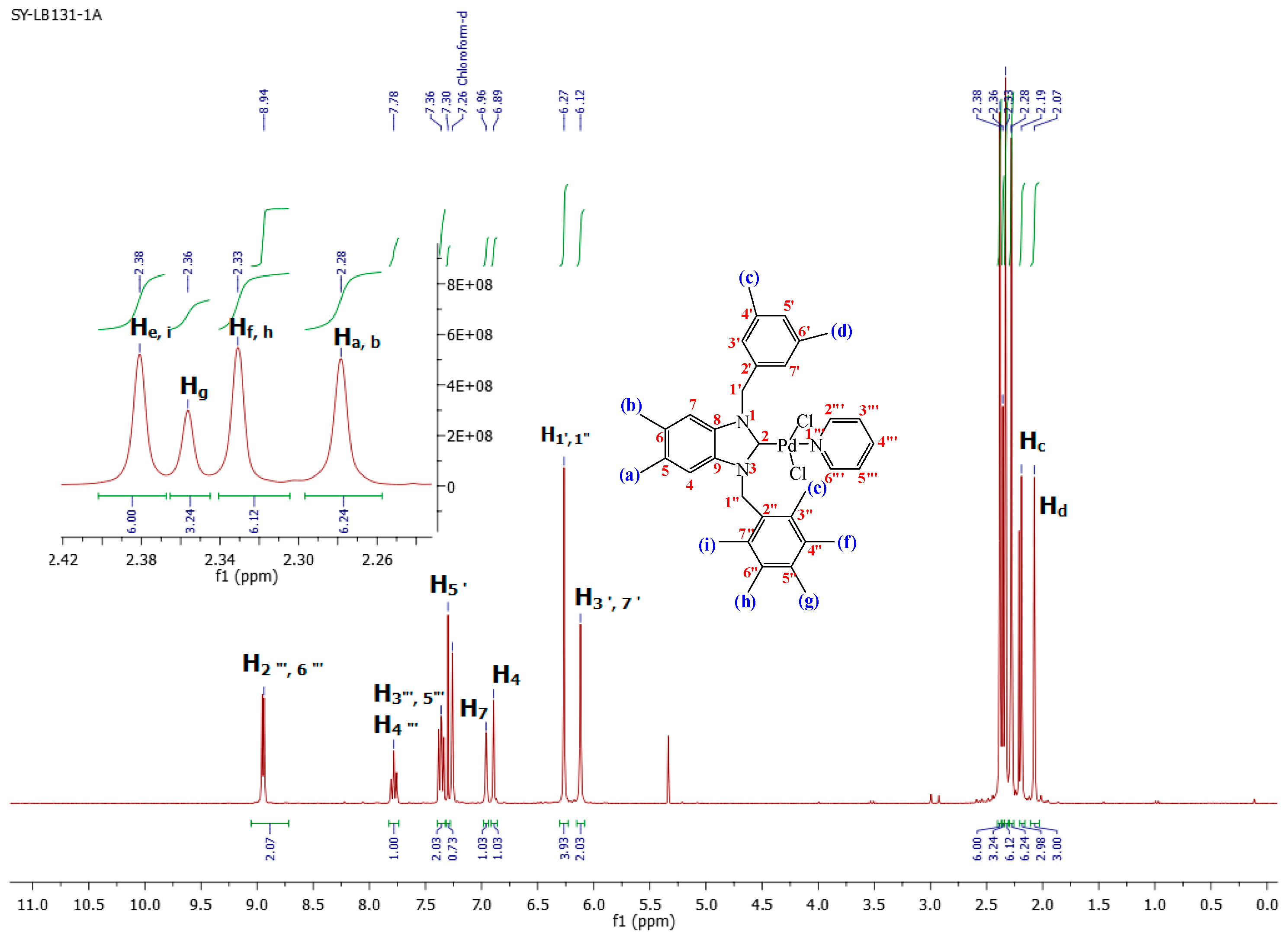
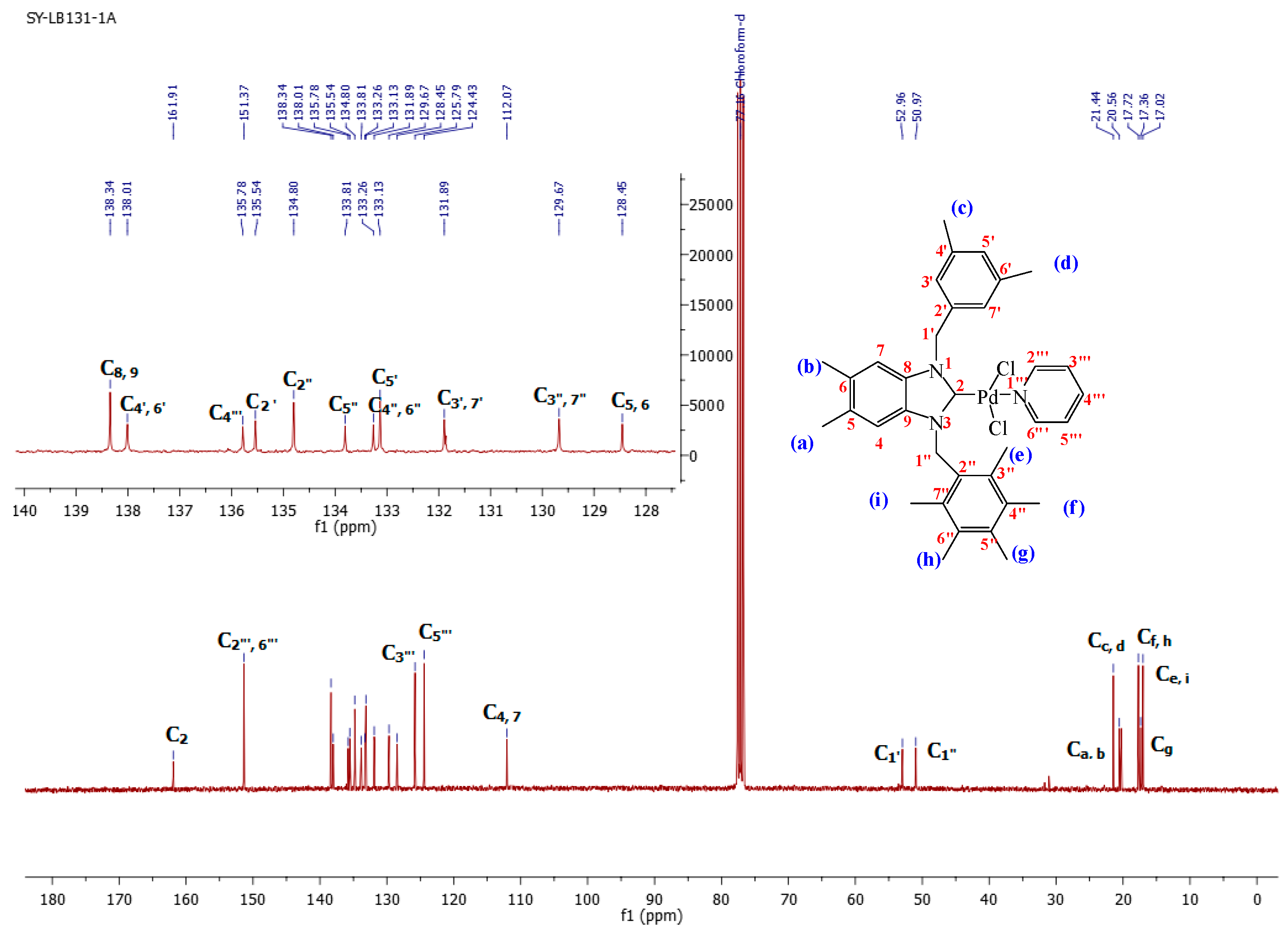
1-(3,5-Dimethylbenzyl)-5,6-dimethyl-3-(pentamethylbenzyl)-benzimidazoliN-2-ylidene-N-(pyridine)dichloro palladium (II) complex (3a):Yield: 92(%). M.p. = 215 °C. FT-IR (KBr) ν, cm−1: 3062 (C-Harom); 1461 (C-N); 1H-NMR (CDCl3) δ (ppm): 8.94 (dd, 2H, (arom. CH (C2′′′,6′′′); 7.78 (m, 1H, (arom. CH (H4′′′)); 7.36 (m, 2H, (arom. CH (H3′′′,5′′′); 7.30 (s, 1H, (arom. CH (H5′); 6.96 (s, 1H, (arom.CH (H7); 6.89 (s, 1H, (arom. CH (H4); 6.27 (s, 4H, (2× CH2 (H1′,1′′); 6.12 (s, 2H, (arom. CH (H3′,7′); 2.38 (s, 6H, 2× CH3 (He, i); 2.36 (s, 3H, (CH3 (Hg); 2.33 (s, 6H, (2× CH3 (Hf,h); 2.28 (s, 6H, (2× CH3 (Ha, b); 2.19 (s, 3H, (CH3 (Hc); 2.07 (s, 3H, (CH3 (Hd). 13C-NMR (CDCl3) δ (ppm): 161.91 (NCN (C2); 151.37 (arom. CH (C2′′′,6′′′); 138.34 (arom. Cq (C8,9); 138.01 (arom. Cq (C4′,6′); 135.78 (arom. CH (C4′′′); 135.54 (arom. Cq (C2′); 134.80 (arom. Cq (C2′′); 133.81 (arom. Cq (C5′′); 133.26 (arom. Cq (C4′′,6′′); 133.13 (arom. CH (C5′); 131.89 (arom. CH (C3′,7′); 129.67 (arom. Cq (C3′′,7′′); 128.45 (arom. Cq (C5,6); 125.79–124.43 (arom. CH (C3′′′,5′′′); 112.07 (arom. CH (C4,7); 52.96 (CH2 (C1′); 50.97 (CH2 (C1′′); 21.44 (CH3 (Cc,d); 20.56 (CH3 (Ca,b); 17.32 (CH3 (Cf,h); 17.36 (CH3 (Cg); 17.02 (CH3 (Ce,i). (DART-TOF-MS) (m/z = 520.3). Anal. Calc. for C35H42N3PdCl2: C, 61.634%; H, 6.207%; N, 6.161%, Found: C, 61.7; H, 6.3; N, 6.2%.
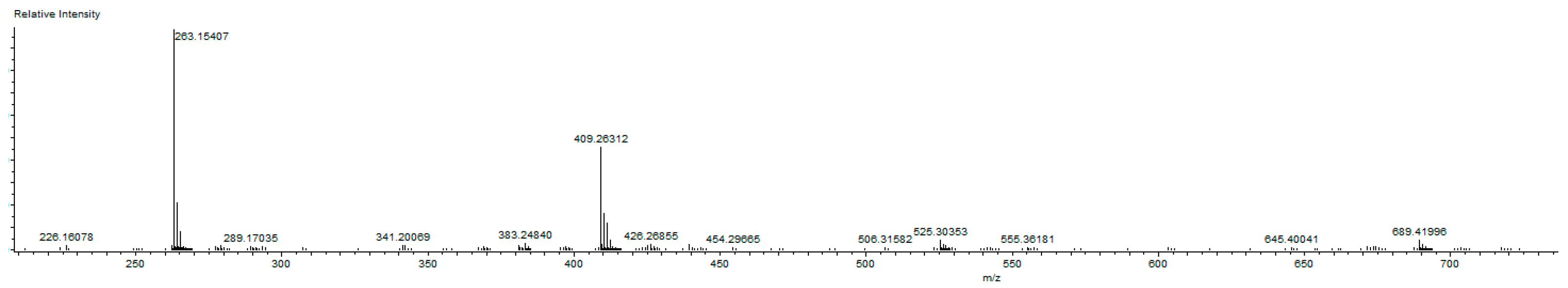
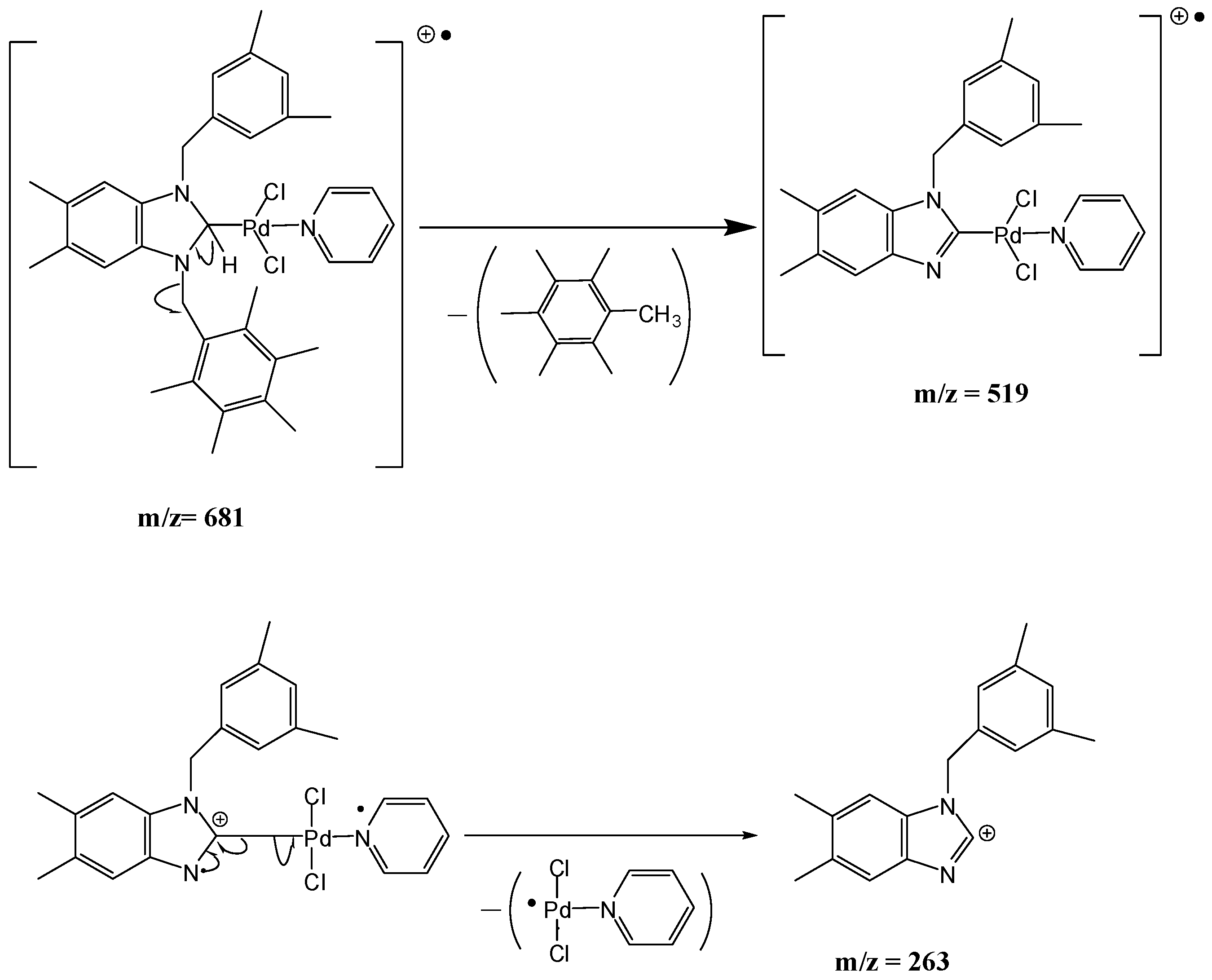
1-(3,5-Dimethylbenzyl)-3-(cyanobenzyl)-5,6-dimethylbenzimidazol-2-ylidene-N-(pyridine)dichloro palladium (II) complex (3b). Yield: 85(%). M.p. = 225 °C. FT-IR (KBr) ν, cm−1: 3062 (C-Harom); 1463 (C-N); 1H-NMR (CDCl3) δ (ppm): 8.94 (d, 2H, H2′′′,6′′′, arom. CH); 7.76 (m, 1H, H4′′′, arom. CH); 7.66 (s, 4H, H3′′,4′′,6′′,7′′, arom. CH); 7.33 (m, 2H, H3′′′,5′′′, arom. CH); 7.24 (s, 1H, H4,7, arom. CH); 6.96(s, 1H, H3′,7′, arom. CH); 6.78 (s, 1H, H5′, arom. CH); 6.24 (s, 2H, H1′, CH2); 6.08 (s, 2H, H1′′, CH2); 2.30 (s, 6H, Hc,d, 2× CH3); 2.22 (s, 6H, Ha,b, 2× CH3). 13C-NMR (CDCl3) δ (ppm): 163.36 (NCN (C2); 151.37 (arom. CH (C2′′′,6′′′); 140.94 (arom. Cq (C8,9); 138.57 (arom. Cq (C2′′); 138.32 (arom. Cq (C4′,6′); 135.10 (arom. CH (C4′′′); 133.37 (arom. Cq (C2′); 133.01 (arom. CH (C4′′,6′′); 132.83 (arom. CH (C5′); 129.96 (arom. CH (C3′′,7′′); 128.53 (arom. CH (C3′,7′); 125.79 (arom. Cq (C5,6); 124.64 (arom. CH (C3′′′,5′′′); 118.77 (CN); 112.05 (arom. CH (C4,7); 111.10 (arom. Cq (C5′′); 52.91 (CH2 (C1′); 52.25 (CH2 (C1′′); 21.44 (CH3 (Cc,d); 20.40 (CH3 (Ca,b). (DART-TOF-MS) (m/z = 523.3, m/z = 263.1). Anal. Calc. for C31H31N4PdCl2: C, 58.458 %; H, 4.906 %; N, 8.796 %, Found: C, 58.7; H, 5.1; N, 8.8%.
1-(3,5-Dimethylbenzyl)-5,6-dimethyl-3-(2-methylbenzyl)-benzimidazoli-2-yl-idene-N-(pyridine)dichloro palladium (II) complex(3c). Yield: 90(%). M.p. = 235 °C. FT-IR (KBr) ν, cm−1: 3061 (C-Harom); 1460 (C-N); 1H-NMR (CDCl3) δ (ppm): 8.91 (s, 2H, H2′′′,6′′′, arom. CH), 7.71 (t, 1H, H4′′′, arom. CH); 7.28 (m, 2H, H3′′′,5′′′, arom. CH); 7.24 (s, 1H, H3′′, arom. CH); 7.22 (s, 3H, H4′′,5′′,6′′, arom. CH); 7.20 (s, 1H, H5′, arom. CH); 6.92 (s, 2H, H3′,7′, arom. CH); 6.71 (s, 2H, H4,7, arom. CH); 6.13 (s, 2H, H1′, CH2); 6.08 (s, 2H, H1′′, CH2); 2.49 (s, 3H, He, CH3); 2.28(s, 6H, Hc,d, 2× CH3); 2.19 (s, 3H, Ha, CH3); 2.16 (s, 3H, Hb, CH3). 13C-NMR (CDCl3) δ (ppm): 162.84 (NCN (C2); 151.44 (arom. CH (C2′′′,6′′′); 138.46 (arom. Cq (C8,9); 138.10 (arom. Cq (C4′,6′); 135.53 (arom. CH (C4′′′); 133.50 (arom .Cq (C2′); 133.26 (arom. Cq (C2′′); 132.55 (arom. Cq (C7′′); 130.45 (arom. CH (C6′′); 129.80 (arom. CH (C5′); 128.12 (arom. CH (C5′′); 127.92 (arom. CH (C3′,7′); 126.61 (arom. CH (C3′′); 125.76 (arom. Cq (C5,6); 124.49 (arom. CH (C4′′); 111.77 (arom. CH (C3′′′,5′′′); 111.49 (arom. CH (C4,7); 52.88 (CH2 (C1′); 50.30 (CH2 (C1′′); 21.44 (CH3 (Cc,d); 20.41(CH3 (Ce); 19.89 (CH3 (Ca,b). (DART-TOF-MS) (m/z = 464.26, m/z = 384.2). Anal. Calc. for C31H34N3PdCl2: C, 59.483%; H, 5.475 %; N, 6.713%, Found: C, 59.5; H, 5.6; N, 6.8%.
3.6. General Procedure for the Suzuki Miyaura Reaction
Phenylboronic acid (0.75 mmol), aryl halides (0.5 mmol), palladium catalyst (0.25 mol %), base (1 mmol) and solvent (1:1) (6 mL) were added under argon to a Schlenk flask containing a magnetic stir bar. The mixture was vigorously stirred at 80 °C for the indicate time. Upon completion, the mixture was cooled to room temperature, extracted with ethyl acetate (5 mL) and filtered through a short pad of silica gel. The filtrate was sampled at intervals for GC analysis.
3.7. Antibacterial Activity
3.7.1. Bacterial Strains, Media and Growth Conditions
Bacteria strains, Gram-positive bacteria: Micrococcus luteus LB 14110, Staphylococcus aureus ATCC 6538 and Listeria monocytogenes ATCC 19117, and Gram-negative bacteria: Salmonella Typhimurium ATCC 14028 and Pseudomonas aeruginosa ATCC 49189, used as indicator microorganisms for the antibacterial activity assays, were obtained from International Culture Collections (ATCC) and local culture collection of Laboratory of Microorganisms and Biomolecules of the Centre of Biotechnology of Sfax-Tunisia. For antibacterial determination, indicator microorganisms were grown overnight in Luria-Bertani (LB) agar medium composed of (g/L): peptone 10; yeast extract 5; and NaCl 5 at pH 7.2 under aerobic conditions and constant agitation (200 rpm) at 30 °C for M. luteus LB14110, and L. monocytogenes ATCC 19117 and at 37 °C for S. aureus ATCC 6538, S. Typhimurium ATCC 14028 and P. aeruginosa ATCC 49189, and then diluted 1:100 in LB media and incubated for 5 h under constant agitation (200 rpm) at the appropriate temperature.
3.7.2. Agar Well Diffusion Method
Agar well diffusion method was employed for the determination of the antibacterial activity of the synthesized compounds with some modifications according to [
23].
3.7.3. MIC Determination
The antimicrobial activities of the synthesized compounds were determined by the minimum inhibitory concentration (MICs) in accordance with NCCLS guideline M7-A
6 and M38-P [
24]. The test was performed in sterile 96-well microplates with a final volume in each microplate well of 100 μL. The synthesized compounds (20 mg/mL) were properly prepared in solution of dimethylsulfoxide (DMSO)/water (1/9;
v/
v). The inhibitory activity of each synthesized compound was transferred to each well in order to obtain a twofold serial dilution of the original sample and to produce the concentration range of 0.0048–20 mg/mL.
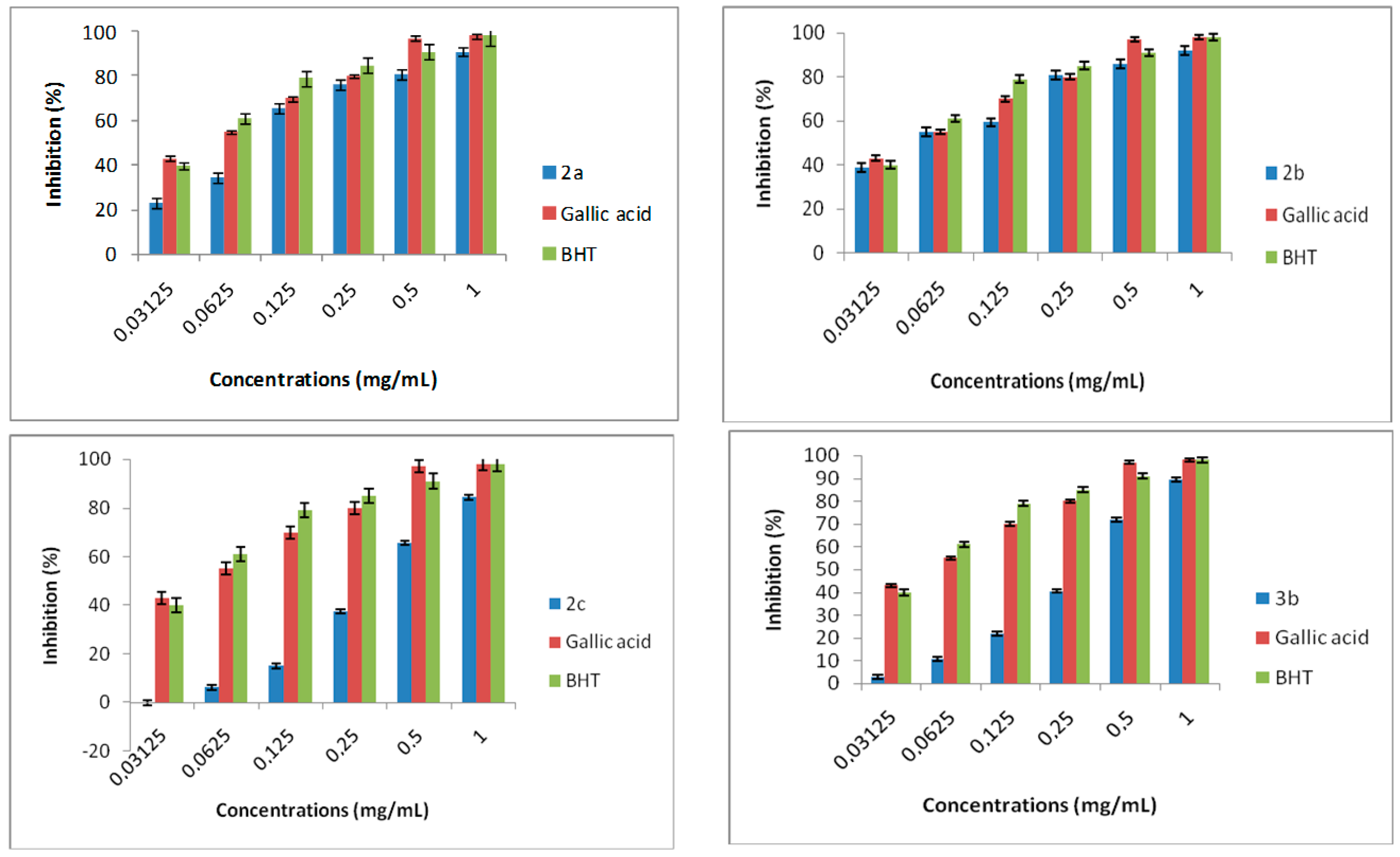
3.8. DPPH Radical Scavenging Activity
DPPH possess a proton free radical, when DPPH encounters proton radical scavengers its purple color fades rapidly. This assay determines the scavenging of stable radical species according to the method of [
25], with slight modifications. Briefly, synthesized compounds were dissolved in dimethylsulfoxide (DMSO)/water (1/9;
v/
v) and diluted with ultrapure water at different concentrations (1, 0.5, 0.250, 0.125, 0.0625, 0.03125 mg/mL). Then, 500 μL of a 4% (
w/
v) solution of DPPH radical in methanol was mixed with 500 μL of samples. The mixture was incubated for 30 min in the dark at room temperature. The scavenging capacity was determined spectrophotometrically by monitoring the decrease in absorbance at 517 nm against a blank. The percentage of antiradical activity (% ArA) had been calculated as follows: % ArA = [(absorbance of control − absorbance of test sample)/absorbance of control] × 100. All tests are assayed in triplicate and expressed as the average ± standard deviation of the measurements.
3.9. Acetylcholinesterase Inhibitory Potential
AChE inhibitory activity was measured by slightly modified spectrophotometric method of Ellman et al.[
26]. Electric eel AChE was used, while acetylthiocholine iodide (ATCI) was employed as substrate of the reaction. 5.5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was used for the measurement of the antiacetylcholinesterase activity. Briefly, in this method, 100 μL of Tris buffer at 50 mM (pH 8.0), 30 μL of sample or standard and 5 μL of AChE enzyme (0.5 U/mL) were added in a 96 well microplate and incubated for 10 min at 25 °C. Then, 142 μL of DTNB (3 mM) and 23 μL of substrate (75 mM) were added. Percentage of inhibition of AChE was determined by comparison of rates reaction of samples relative to control (10% DMSO in Tris buffer) using the following formula:
where δA sample: Sample absorbance at zero time − Sample absorbance at the end of reaction, and δA control: Control absorbance at zero time − Control absorbance at the end of reaction. Galanthamine, an antiacetylcholinesterase alkaloid type of drug obtained from the snowdrop bulbs (
Galanthus sp.), was used as standard. All synthesized compounds have been tested at 100 μg/mL of concentration. This determination was done in triplicate and obtained results were very similar. The reported value is the average of the three tests.