Valorization of Phosphorus Secondary Raw Materials by Acidithiobacillus ferrooxidans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changes in pH
2.2. Concentration of Phosphorus (Expressed as P2O5)
2.3. Solubilization of Apatite
3. Materials and Methods
3.1. Microorganisms
3.2. Solubilization Experiments
3.3. Analytical Methods
3.4. Calculations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Besharati, H.; Atashnama, K.; Hatami, S. Biosuper as a phosphate fertilizer in a calcareous soil with low available phosphorus. Afr. J. Biotechnol. 2007, 6, 1325–1329. [Google Scholar]
- Malinowski, P.; Olech, M.; Sas, J.; Wantuch, W.; Biskupski, A.; Urbańczyk, L.; Borowik, M.; Kotowicz, J. Production of compound mineral fertilizers as a method of utilization of waste products in chemical company Alwernia S.A. Pol. J. Chem. 2010, 12, 6–9. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Chojnacka, K. In situ solubilization of phosphorus bearing raw materials by Bacillus megaterium. Eng. Life Sci. 2017. [Google Scholar] [CrossRef]
- Hayat, R.; Safdar, A.; Ummay, A.; Rabia, K.; Iftikhar, A. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.-A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Santos, N.P.; Moura, P.R.; Freire, A.M.M. Biofertilizers with natural phosphate, sulphur and Acidithiobacillus in a soil with low available-P. Sci. Agricola 2003, 60, 767–773. [Google Scholar]
- Wyciszkiewicz, M.; Saeid, A.; Samoraj, M.; Chojnacka, K. Solid-state solubilization of bones by B. megaterium in spent mushroom substrate as a medium for a phosphate enriched substrate. J. Chem. Technol. Biotechnol. 2016. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Górecki, H.; Chojnacka, K. New generation of phosphate fertilizer from bones, produced by bacteria. Open Chem. 2015, 13, 951–958. [Google Scholar] [CrossRef]
- Coates, L.R. Method of Producing Fertilizers. Patent US947798, 1 February 1910. [Google Scholar]
- Du, S.Q.; DU, S.W.; Qin, Z.W. Process for Preparing biologic Organic and Mineral Compound Fertilizer. Patent Apl. CN1262260, 9 August 2000. [Google Scholar]
- Wang, D.Z.; Wang, L. Three microbe Granular Fertilizer and Producing Process Thereof. Patent Apl. CN1175565, 11 March 1998. [Google Scholar]
- Liu, W. Method for Producing Organic and Inorganic Mixed Microbe Fertilizer. Patent CN1054362, 12 July 2000. [Google Scholar]
- Rajan, S.S.S. Use of low grade phosphate rocks as biosuper fertilizer. Fertil. Res. 1981, 2, 199–209. [Google Scholar] [CrossRef]
- Kucera, J.; Pakostova, E.; Lochman, J.; Janiczek, O.; Mandl, M. Are there multiple mechanisms of anaerobic sulfur oxidation with ferric iron in Acidithiobacillus ferrooxidans? Res. Microbiol. 2016, 167, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Guo, C.; Zhang, T.; Lu, G.; Wan, J.; Liao, C.; Dang, Z. Investigation of intermediate sulfur species during pyrite oxidation in the presence and absence of Acidithiobacillus ferrooxidans. Hydrometallurgy 2017, 167, 58–65. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Tao, J.; Feng, X.; Liu, X.; Qin, W. Differential fluoride tolerance between sulfur- and ferrous iron-grown Acidithiobacillus ferrooxidans and its mechanism analysis. Biochem. Eng. J. 2017, 119, 59–66. [Google Scholar] [CrossRef]
- Martínez-Bussenius, C.; Navarro, C.A.; Orellana, L.; Paradela, A.; Jerez, C.A. Global response of Acidithiobacillus ferrooxidans ATCC 53993 to high concentrations of copper: A quantitative proteomics approach. J. Proteom. 2016, 145, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Nguyen, T.A.H.; Taran, E.; Mahler, S.M.; Nguyen, A.V. Effect of energy source, salt concentration and loading force on colloidal interactions between Acidithiobacillus ferrooxidans cells and mineral surfaces. Colloids Surf. B. Biointerfaces 2015, 132, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Avdalović, J.; Beškoski, V.; Gojgić-Cvijovića, G.; Mattinenc, M.-L.; Stojanovićd, M.; Zildžovićd, S.; Vrvić, M.M. Microbial solubilization of phosphorus from phosphate rock by iron-oxidizing Acidithiobacillus sp. B2. Miner. Eng. 2015, 72, 17–22. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil-phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the. Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Xiao, C.X.; Chi, R.-A.; Fang, Y.-J. Effects of Acidiphilium cryptum on biosolubilization of rock phosphate in the presence of Acidithiobacillus ferrooxidans. Trans. Nonferrous Meter. Soc. China. 2013, 23, 2153–2159. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Dobrowolska-Iwanek, J.; Chojnacka, K. Utilization of microorganisms in the solubilization of low-quality phosphorus raw material. Ecol. Eng. 2016, 89, 109–113. [Google Scholar] [CrossRef]
- Saeid, A.; Labuda, M.; Chojnacka, K.; Górecki, H. Valorization of Bones to Liquid Phosphorus Fertilizer by Microbial Solubilization. Waste Biomass Valori. 2014, 5, 265–272. [Google Scholar] [CrossRef]
- Phiraphinyo, P.; Taepakpurenat, S.; Lakkanatinaporn, P.; Suntornsuk, W.; Suntornsuk, L. Physical and chemical properties of fish and chicken bones as calcium source for mineral supplements. J. Sci. Technol. 2006, 28, 327–335. [Google Scholar]
- Wyciszkiewicz, M.; Saeid, A.; Chojnacka, K.; Górecki, H. Production of phosphate biofertilizers from bones by phosphate-solubilizing bacteria Bacillus megaterium. Open Chem. 2015, 13, 1063–1070. [Google Scholar] [CrossRef]
- Bhatti, T.M.; Yawar, W. Bacterial solubilization of phosphorus from phosphate rock containing sulfur-mud. Hydrometallurgy. 2010, 103, 54–59. [Google Scholar] [CrossRef]
- Mendes, G.O.; Vassilev, N.B.; Bonduki, V.H.A.; Silva, I.R.; Ribeiro, J.I.; Costa, D. Inhibition of Aspergillus niger Phosphate Solubilization by Fluoride Released from Rock Phosphate. Appl Environ Microbiol. 2013, 16, 4906–4913. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Strother, P.; McMahon, G.; Nittler, L.R.; Wang, J.; Zhang, J.; Sang, L.; Ma, Ch.; Papineau, D. Terminal Proterozoic cyanobacterial blooms and phosphogenesis documented by the Doushantuo granular phosphorites I: In situ micro-analysis of textures and composition. Precambrian Res. 2013, 235, 20–35. [Google Scholar] [CrossRef]
- Mendes, G.O.; Zafra, D.L.; Vassilev, N.B.; Silva, I.R.; Ribeiro, J.I.; Costa, M.D. Biochar Enhances Aspergillus niger Rock Phosphate Solubilization by Increasing Organic Acid Production and Alleviating Fluoride Toxicity. Appl. Environ. Microbiol. 2014, 80, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Chi, R.; Xiao, C.; Gao, H. Bioleaching of phosphorus from rock phosphate containing pyrites by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 979–981. [Google Scholar] [CrossRef]
- Tutken, T.; Vennemann, T.W.; Pfretzschner, H.-U. Early diagenesis of bone and tooth apatite in fluvial and marine settings: Constraints from combined oxygen isotope, nitrogen and REE analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 266, 254–268. [Google Scholar] [CrossRef]
- Soudry, D.; Nathan, Y. Microbial infestation: A pathway of fluorine enrichment in bone apatite fragments (Negev phosphorites, Israel). Sediment. Geol. 2000, 132, 171–176. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; AbdelKareem, H.N. Sustainable approach for recycling waste lamb and chicken bones for fluoride removal from water followed by reusing fluoride-bearing waste in concrete. Waste Manag. 2015, 45, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yield. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [PubMed]
- Górecka, H.; Chojnacka, K.; Górecki, H. The application of ICP-MS and ICP-OES in determination of micronutrients in wood ashes used as soil conditioners. Talanta 2006, 70, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
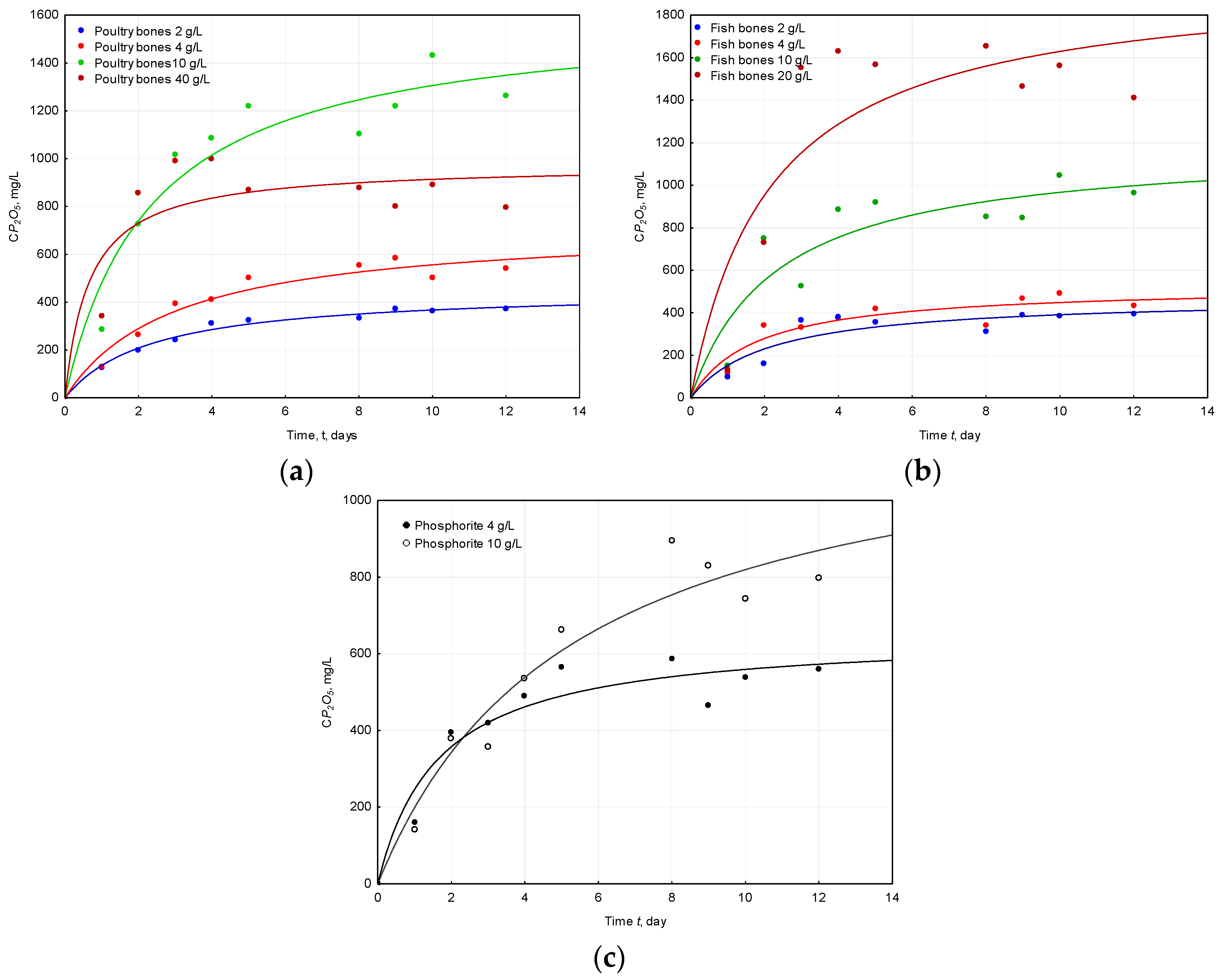
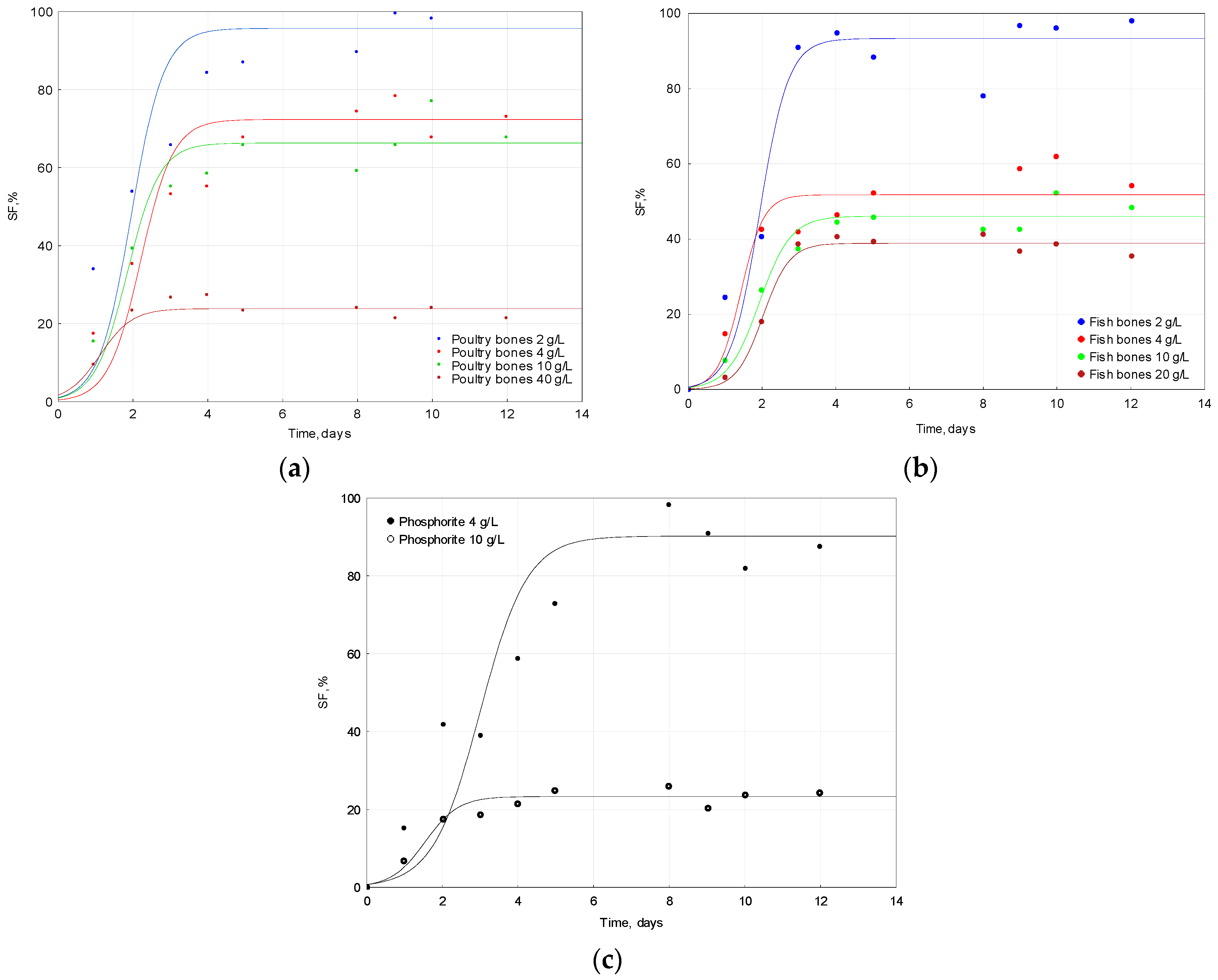
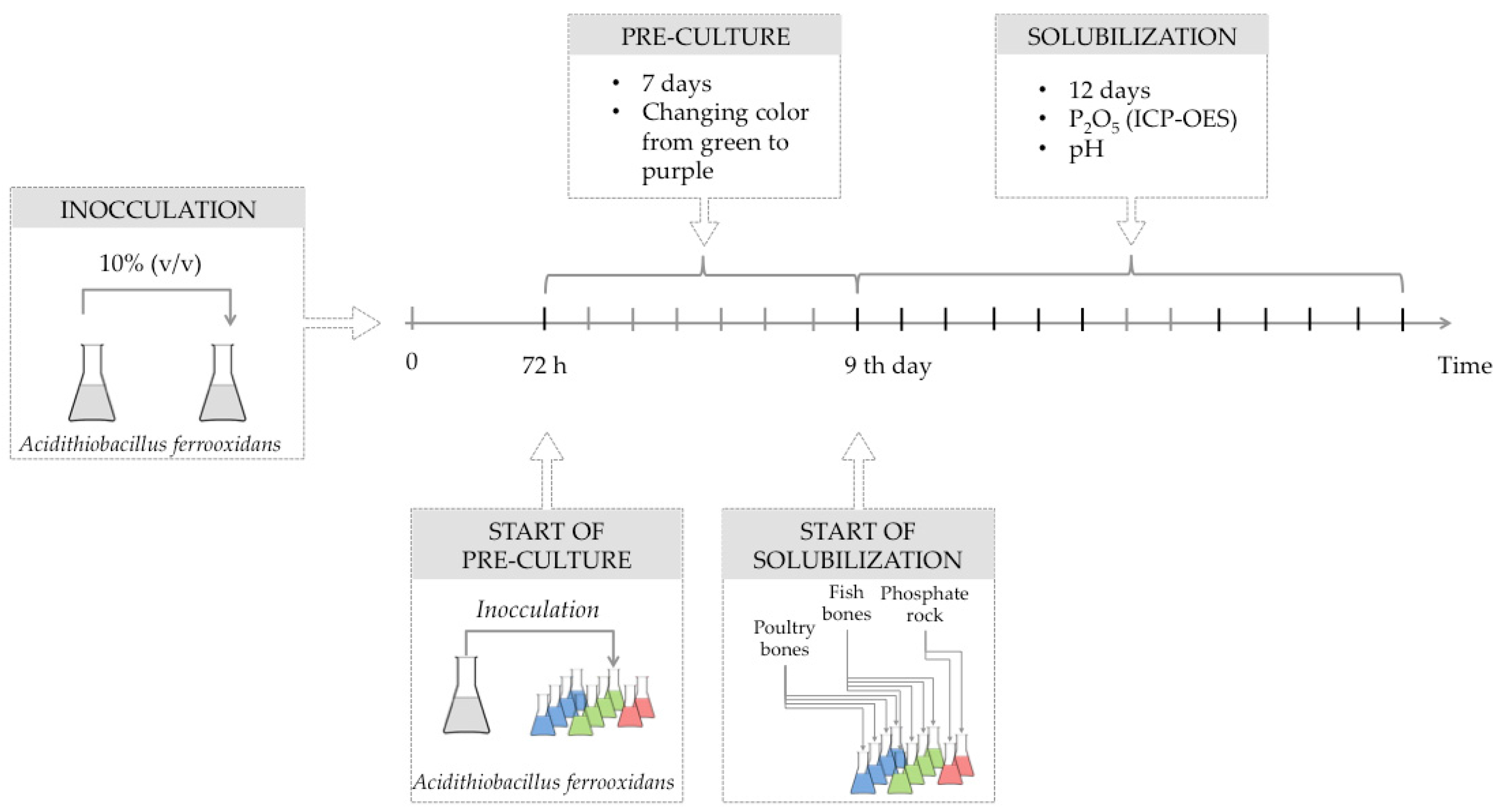
| Raw Material | Dose | pH Initial | pH Final | ΔpH |
|---|---|---|---|---|
| POULTRY BONES | 2 | 2.495 | 1.65 | 0.845 |
| 4 | 2.529 | 1.744 | 0.785 | |
| 10 | 2.484 | 1.832 | 0.652 | |
| 20 | 2.518 | 1.895 | 0.623 | |
| FISH BONES | 2 | 2.51 | 1.753 | 0.757 |
| 4 | 2.489 | 1.7 | 0.789 | |
| 10 | 2.497 | 1.643 | 0.854 | |
| 20 | 2.498 | 1.637 | 0.861 | |
| PHOSPHATE ROCK | 4 | 2.494 | 1.853 | 0.641 |
| 10 | 2.528 | 2.720 | −0.192 |
| RM | Dose g/L | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Value | SE | p | R2 | χ2 | Parameter | Value | SE | p | R2 | χ2 | ||
| POULTRY BONES | 2 | CmaxP2O5, mg/L | 454 | 18.1 | 0.000 | 0.993 | 6.17 | SFmax, % | 95.7 | 3.4 | 0.000 | 0.982 | 16.1 |
| K, 1/day | 0.424 | 0.056 | 0.000 | k, 1/day | 0.418 | 0.078 | 0.001 | ||||||
| L, day | 1.95 | 0.21 | 0.000 | ||||||||||
| 4 | CmaxP2O5, mg/L | 722 | 58 | 0.000 | 0.981 | 36.54 | SFmax, % | 72.4 | 2.5 | 0.000 | 0.985 | 9.30 | |
| K, 1/day | 0.335 | 0.0805 | 0.00315 | k, 1/day | 0.435 | 0.077 | 0.001 | ||||||
| L, day | 2.21 | 0.20 | 0.000 | ||||||||||
| 10 | CmaxP2O5, mg/L | 1614 | 148 | 0.000 | 0.968 | 150.5 | SFmax, % | 66.3 | 2.4 | 0.000 | 0.980 | 7.49 | |
| K, 1/day | 0.424 | 0.129 | 0.0111 | k, 1/day | 0.619 | 0.146 | 0.004 | ||||||
| L, day | 1.85 | 0.19 | 0.000 | ||||||||||
| 20 | CmaxP2O5, mg/L | 975 | 98 | 0.000 | 0.904 | 231 | SFmax, % | 23.9 | 0.8 | 0.000 | 0.978 | 1.47 | |
| K, 1/day | 1.49 | 0.98 | 0.164 | k, 1/day | 1.74 | 1.32 | 0.229 | ||||||
| CmaxP2O5, mg/L | L, day | 1.12 | 0.12 | 0.000 | |||||||||
| FISH BONES | 2 | K, 1/day | 474 | 63 | 0.000 | 0.931 | 95.1 | SFmax, % | 93.9 | 4.0 | 0.000 | 0.972 | 15.9 |
| CmaxP2O5, mg/L | 0.474 | 0.220 | 0.0632 | k, 1/day | 0.784 | 0.248 | 0.016 | ||||||
| K, 1/day | L, day | 1.94 | 0.20 | 0.000 | |||||||||
| 4 | CmaxP2O5, mg/L | 529 | 53 | 0.000 | 0.950 | 62.3 | SFmax, % | 51.8 | 2.8 | 0.000 | 0.949 | 9.18 | |
| K, 1/day | 0.560 | 0.213 | 0.0304 | k, 1/day | 0.891 | 0.373 | 0.048 | ||||||
| CmaxP2O5, mg/L | L, day | 1.42 | 0.25 | 0.001 | |||||||||
| 10 | K, 1/day | 1189 | 169 | 0.000 | 0.929 | 286 | SFmax, % | 46.1 | 1.4 | 0.000 | 0.961 | 3.61 | |
| CmaxP2O5, mg/L | 0.435 | 0.205 | 0.0664 | k, 1/day | 0.716 | 0.155 | 0.002 | ||||||
| K, 1/day | L, day | 1.92 | 0.15 | 0.000 | |||||||||
| 20 | CmaxP2O5, mg/L | 1980 | 342 | 0.000 | 0.898 | 763 | SFmax, % | 38.9 | 0.9 | 0.000 | 0.992 | 3.36 | |
| K, 1/day | 0.464 | 0.277 | 0.132 | k, 1/day | 1.403 | 0.492 | 0.025 | ||||||
| CmaxP2O5, mg/L | L, day | 2.02 | 0.07 | 0.000 | |||||||||
| PHOSPHATE ROCK | 4 | K, 1/day | 1256 | 187 | 0.000 | 0.971 | 86.3 | SFmax, % | 90.8 | 4.5 | 0.000 | 0.971 | 7.29 |
| CmaxP2O5, mg/L | 0.188 | 0.0637 | 0.0186 | k, 1/day | 0.292 | 0.072 | 0.007 | ||||||
| K, 1/day | L, day | 2.96 | 0.33 | 0.000 | |||||||||
| 10 | CmaxP2O5, mg/L | 651 | 55 | 0.000 | 0.961 | 65.3 | SFmax, % | 23.7 | 1.0 | 0.000 | 0.945 | 1.45 | |
| K, 1/day | 0.607 | 0.203 | 0.0171 | k, 1/day | 0.532 | 0.170 | 0.020 | ||||||
| L, day | 1.52 | 0.24 | 0.001 | ||||||||||
| K | k | SF | L | ||
|---|---|---|---|---|---|
| 1.00 | |||||
| K | −0.095 | 1.00 | |||
| K | 0.314 | 0.794 ** | 1.00 | ||
| SF | −0.269 | −0.618 * | −0.628 * | 1.00 | |
| L | 0.261 | −0.778 ** | −0.618 * | 0.665 ** | 1.00 |
| Formulations | Cd | Cu | Cr | Ni | Pb | Zn |
|---|---|---|---|---|---|---|
| Poultry Bone | 0.0874 a ± 0.1095 | 0.239 b ± 0.473 | 0.450 c ± 1.991 | 3.81 k ± 1.26 | 2.67 ± 2.16 | 2.58 d ± 2.33 |
| Fish Bone | 0.104 e ± 0.089 | 0.253 f ± 0.221 | 0.0214 g ± 0.0506 | 5.05 h,k ± 2.06 | 3.04 ± 2.14 | 3.51 j ± 2.29 |
| Phosphorite | 0.260 a,e ± 0.167 | 0.671 b,f ± 1.087 | 2.66 c,g ± 5.12 | 3.54 h ± 1.46 | 3.39 ± 3.75 | 7.36 d,j ± 4.24 |
| Acceptable limits | 3 mg/kg | 400 mg/kg | 100 mg/kg | 30 mg/kg | 100 mg/kg | 1500 mg/kg |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyciszkiewicz, M.; Saeid, A.; Malinowski, P.; Chojnacka, K. Valorization of Phosphorus Secondary Raw Materials by Acidithiobacillus ferrooxidans. Molecules 2017, 22, 473. https://doi.org/10.3390/molecules22030473
Wyciszkiewicz M, Saeid A, Malinowski P, Chojnacka K. Valorization of Phosphorus Secondary Raw Materials by Acidithiobacillus ferrooxidans. Molecules. 2017; 22(3):473. https://doi.org/10.3390/molecules22030473
Chicago/Turabian StyleWyciszkiewicz, Małgorzata, Agnieszka Saeid, Przemysław Malinowski, and Katarzyna Chojnacka. 2017. "Valorization of Phosphorus Secondary Raw Materials by Acidithiobacillus ferrooxidans" Molecules 22, no. 3: 473. https://doi.org/10.3390/molecules22030473






