Chemical Constituents of Supercritical Extracts from Alpinia officinarum and the Feeding Deterrent Activity against Tribolium castaneum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Compounds
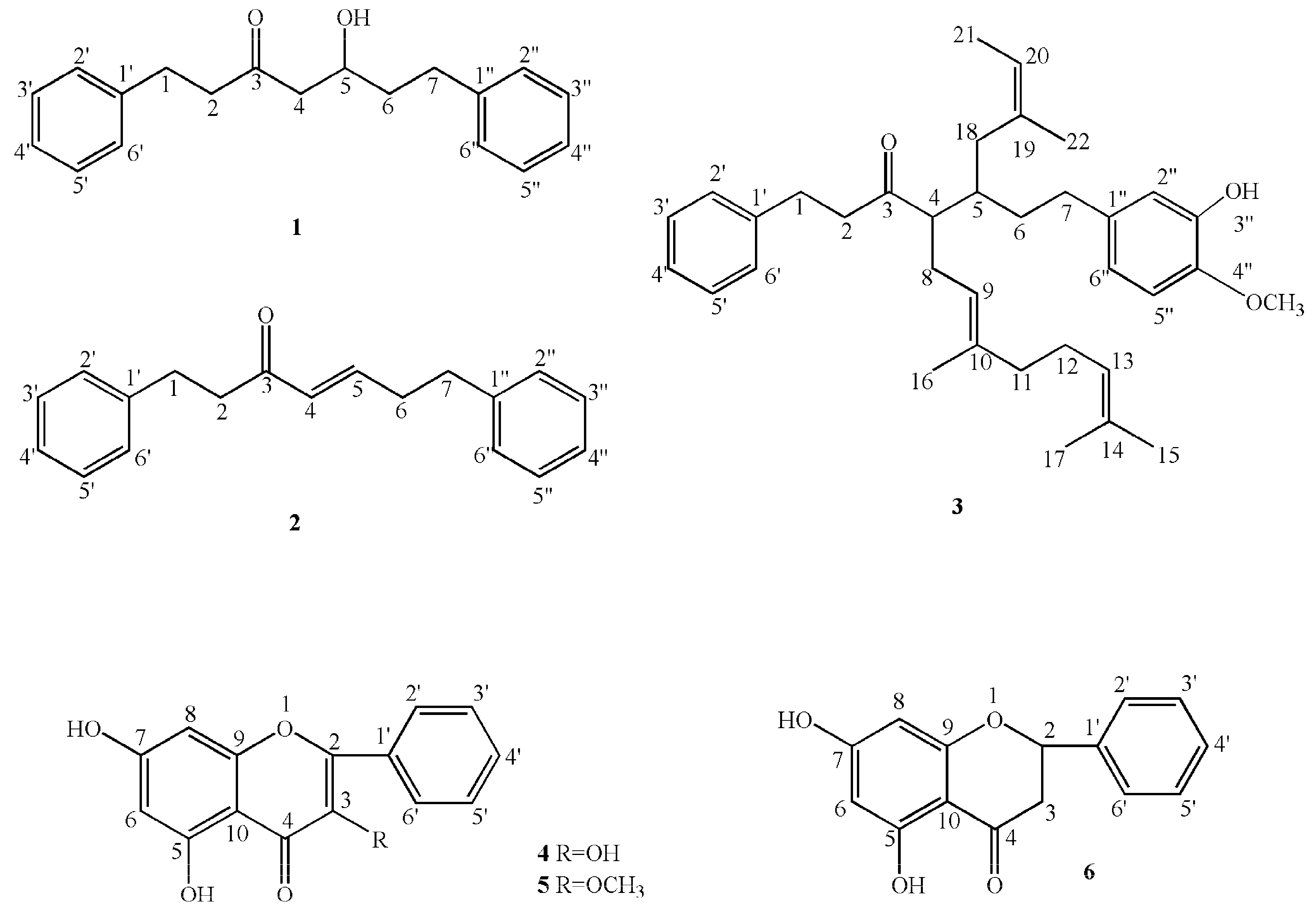
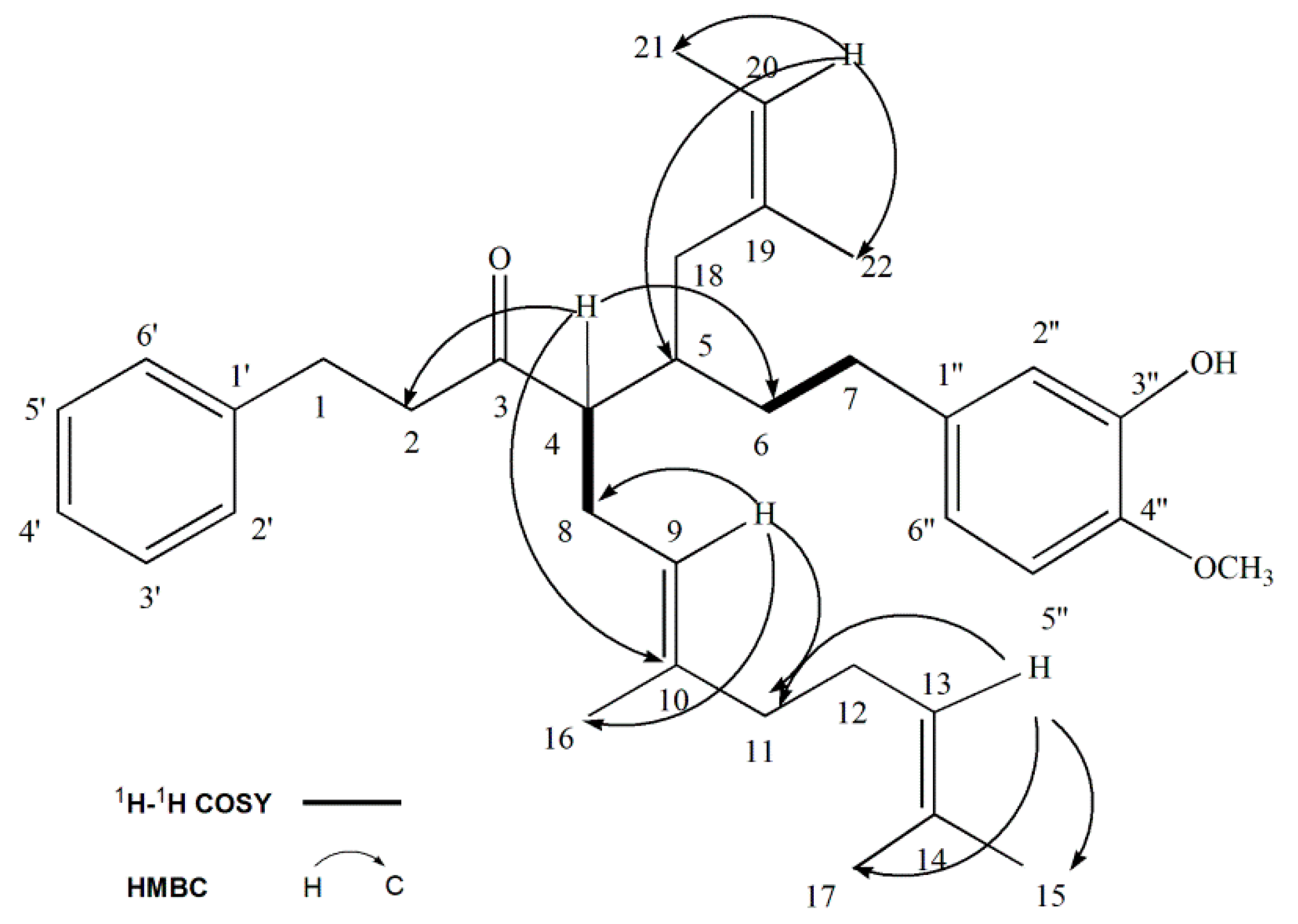
2.2. Structure Elucidation
2.3. Contact Toxicity
2.4. Feeding Deterrent of the Isolated Compounds
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Insects
3.5. GC-MS and GC-FID Analyses
3.6. Identification of the Compounds
3.7. Contact Toxicity
3.8. Feeding Deterrent Bioassay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wijayaratne, L.K.W.; Arthur, F.H.; Whyard, S. Methoprene and control of stored-product insects. J. Stored. Prod. Res. 2016, in press. [Google Scholar] [CrossRef]
- Buckman, K.A.; Campbell, J.F. How varying pest and trap densities affect Tribolium castaneum capture in pheromone traps. Entomol. Exp. Appl. 2013, 146, 404–412. [Google Scholar] [CrossRef]
- Liu, Z.L.; Cao, J.; Zhang, H.M.; Lin, L.L.; Du, S.S.; Zhou, L.G.; Deng, Z.W. Feeding deterrents from Aconitum episcopale roots against the red flour beetle, Tribolium castaneum. J. Agric. Food Chem. 2011, 59, 3701–3706. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Wang, Y.; Zhang, W.J.; Yang, K.; Wu, Y.; Geng, Z.F.; Chen, H.P.; Jiang, H.Y.; Du, S.S.; Deng, Z.W.; et al. Chemical constituents and biological activities of the Purple Perilla essential oil against Lasioderma serricorne. Ind. Crop Prod. 2014, 61, 331–337. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Copping, L.G.; Menn, J.J. Biopesticides: A review of their action, applications and efficacy. Pest Manag. Sci. 2000, 8, 651–676. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Liu, H.; Li, L.S. The main stratagems and technology for stored product pest control in ancient China. J. Southwest Agric. Univ. 2000, 22, 335–338. [Google Scholar]
- Yang, K.; You, C.X.; Wang, C.F.; Guo, S.S.; Li, Y.P.; Wu, Y.; Geng, Z.F.; Deng, Z.W.; Du, S.S. Composition and repellency of the essential oils of Evodia calcicola Chun ex Huang and Evodia trichotoma (Lour.) Pierre against three stored product insects. J. Oleo Sci. 2014, 63, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Yang, K.; You, C.X.; Zhang, W.J.; Guo, S.S.; Geng, Z.F.; Du, S.S.; Wang, Y.Y. Chemical composition and insecticidal activity of essential oils from Zanthoxylum dissitum leaves and roots against three species of storage pests. Molecules 2015, 20, 7990–7999. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.Y.; Wu, H.F.; Tang, Y.L.; Chen, D.Z. A new labdane diterpene from the rhizomes of Alpinia officinarum. Nat. Prod. Res. 2016, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Peng, C.C.; Yeh, X.Y.; Huang, B.Y.; Wang, H.E.; Chen, K.C.; Peng, R.Y. Antihyperlipidemic bioactivity of Alpinia officinarum (Hance) Farw Zingiberaceae can be attributed to the coexistance of curcumin, polyphenolics, dietary fibers and phytosterols. Food Funct. 2015, 6, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Li, Y.H.; Wang, Y.; Wei, N.; Tan, Y.F.; Zhang, J.Q. Advances in studies on chemical constituents in Alpiniae officinarum rhizoma and their pharmacological activities. Chin. J. Exp. Tradit. Med. Form. 2014, 20, 236–244. [Google Scholar]
- Liu, C.N. Antagonistic storage method of traditional Chinese medicinal material. J. Chin. Med. Mater. 1987, 4, 36. [Google Scholar] [CrossRef]
- Liu, Z.L.; Goh, S.H.; Ho, S.H. Screening of Chinese medicinal herbs for bioactivity against Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J. Stored Prod. Res. 2007, 43, 290–296. [Google Scholar] [CrossRef]
- Zhao, N.N.; Zhou, L.G.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Evaluation of the toxicity of the essential oils of some common Chinese spices against Liposcelis bostrychophila. Food Control 2012, 26, 486–490. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Li, Z.H.; Wang, C.F.; Wei, J.Y.; Li, X.L.; Wang, P.J.; Zhou, Z.F.; Du, S.S.; Huang, D.Y.; et al. Composition of the essential oil from Alpinia galanga rhizomes and its bioactivity on Lasioderma serricorne. Bull. Insectol. 2014, 67, 247–254. [Google Scholar]
- Abdullah, F.; Subramanian, P.; Ibrahim, H.; Malek, S.N.A.; Lee, G.S.; Hong, S.L. Chemical composition, antifeedant, repellent, and toxicity activities of the rhizomes of galangal, Alpinia galanga against Asian subterranean termites, Coptotermes gestroi and Coptotermes curvignathus (Isoptera: Rhinotermitidae). J. Insect Sci. 2015, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltrán, J.A. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. 2017, 200, 81–86. [Google Scholar] [CrossRef]
- Garcia-Perez, J.S.; Robledo-Padilla, F.; Cuellar-Bermudez, S.P.; Parra-Saldivar, R.; Zavala-Yoe, R.; Ramirez-Mendoza, R.A.; Iqbal, H.M.N. Thermodynamics and statistical correlation between supercritical-CO2, fluid extraction and bioactivity profile of locally available mexican plants extracts. J. Supercrit. Fluid 2017, 122, 27–34. [Google Scholar] [CrossRef]
- Luo, J.; Rui, W.; Jiang, M.; Tian, Q.; Ji, X.; Feng, Y. Separation and identification of diarylheptanoids in supercritical fluid extract of Alpinia officinarum by UPLC-MS-MS. J. Chromatogr. Sci. 2010, 48, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Sunarso, J.; Ismadji, S. Decontamination of hazardous substances from solid matrices and liquids using supercritical fluids extraction: A review. J. Hazard. Mater. 2009, 161, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.H.; He, J.H.; Zeng, R.J.; Zhao, S.J. Comparison of volatile oil in Alpinia officinarum Hance extracted by different methods. Pharm. Biotechnol. 2008, 15, 54–58. [Google Scholar]
- Hema, P.S.; Nair, M.S. Flavonoids and other constituents from the rhizomes of Alpinia calcarata. Biochem. Syst. Ecol. 2009, 37, 52–54. [Google Scholar] [CrossRef]
- Pulkkinen, J.T.; Honkakoski, P.; Peräkylä, M.; Berczi, I.; Laatikainen, R. Synthesis and evaluation of estrogen agonism of diaryl 4,5-dihydroisoxazoles, 3-hydroxyketones, 3-methoxyketones, and 1,3-diketones: A compound set forming a 4d molecular library. J. Med. Chem. 2008, 1, 3562–3571. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Yang, S.L.; Zhou, Z.M.; Xu, L.Z. Flavonoids of Alpinia officinarum. Chin. Tradit. Herb. Drugs 2006, 5, 663–664. [Google Scholar]
- Zhang, W.J.; Yang, K.; You, C.X.; Wang, Y.; Wang, C.F.; Wu, Y.; Geng, Z.F.; Su, Y.; Du, S.S.; Deng, Z.W. Bioactivity of essential oil from Artemisia stolonifera (Maxim.) Komar. and its main compounds against two stored-product insects. J. Oleo Sci. 2015, 64, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Yang, K.; You, C.X.; Wang, C.F.; Geng, Z.F.; Su, Y.; Wang, Y.; Du, S.S.; Deng, Z.W. Contact toxicity and repellency of the essential oil from Mentha haplocalyx Briq.against Lasioderma serricorne. Chem. Biodivers. 2015, 12, 832–839. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Yang, K.; Wu, Y.; Zhang, W.J.; Wang, Y.; Geng, Z.F.; Chen, H.P.; Jiang, H.Y.; Du, S.S.; Deng, Z.W.; et al. Chemical composition and insecticidal activities of the essential oil of Perilla frutescens (L.) Britt. aerial parts against two stored product insects. Eur. Food Res. Technol. 2014, 239, 481–490. [Google Scholar] [CrossRef]
- Russell, G.B.; Bowers, W.S.; Keesing, V.; Niemeyer, H.M.; Sevenet, T.; Vasanthaverni, S.; Wratten, S.D. Patterns of bioactivity and herbivory on Nothofagus species from Chile and New Zealand. J. Chem. Ecol. 2000, 26, 41–56. [Google Scholar] [CrossRef]
- Napal, G.N.D.; Defagó, M.T.; Valladares, G.R.; Sara, M.P. Response of Epilachna paenulata to two flavonoids, pinocembrin and quercetin, in a comparative study. J. Chem. Ecol. 2010, 36, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Georgina, N.; Napal, D.; Carpinella, M.C.; Palacios, S.M. Antifeedant activity of ethanolic extract from Flourensia oolepis and isolation of pinocembrin as its active principle compound. Bioresour. Technol. 2009, 100, 3669–3673. [Google Scholar]
- Huang, A.; Sefton, M.A.; Taylor, D.K. Comparison of the formation of peppery and woody sesquiterpenes derived from α-guaiene and α-bulnesene under aerial oxidative conditions. J. Agric. Food Chem. 2015, 63, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.P. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. J. Am. Soc. Mass Spectrom. 2001, 16, 1902–1903. [Google Scholar]
- Liu, Z.L.; Ho, S.H. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch. and Tribolium castaneum (Herbst). J. Stored Prod. Res. 1999, 35, 317–328. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 339–347. [Google Scholar]
- Xieal, Y.S.; Bodnarykal, R.P.; Fieldsal, P.G. A rapid and simple flour-disk bioassay for testing substance active against stored-product insects. Can. Entomol. 1996, 5, 865–875. [Google Scholar]
- Du, S.S.; Wang, C.F.; Li, J.; Zhang, H.M.; Liu, Q.Z.; Liu, Z.L.; Deng, Z.W. Antifeedant diterpenoids against Tribolium castaneum from the stems and twigs of Ceriops tagal (Rhizophoraceae). Molecules 2011, 16, 6060–6067. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chu, S.S.; Jiang, G.H. Feeding Deterrents from Zanthoxylum schinifolium against two stored-product insects. J. Agric. Food Chem. 2009, 57, 10130–10133. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; You, C.X.; Yang, K.; Guo, S.S.; Geng, Z.F.; Fan, L.; Du, S.S.; Deng, Z.W.; Wang, Y. Antifeedant activities of methanol extracts of four Zanthoxylum species and benzophenanthridines from stem bark of Zanthoxylum schinifolium against Tribolium castaneum. Ind. Crop. Prod. 2015, 74, 407–411. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compounds | Molecular Formula | RI a | Relative Content | Identification Methods c | |
|---|---|---|---|---|---|
| Essential Oil b | SFE Extracts | ||||
| α-Pinene | C10H16 | 940 | 3.26 | - | RI, MS |
| Camphene | C10H16 | 956 | 4.57 | - | RI, MS, Co |
| Sabinene | C10H16 | 976 | 3.65 | 0.14 | RI, MS, Co |
| β-Pinene | C10H16 | 978 | - | 0.09 | RI, MS, Co |
| α-Phellandrene | C10H16 | 1005 | 0.49 | 0.13 | RI, MS |
| β-Phellandrene | C10H16 | 1026 | 3.42 | - | RI, MS |
| 1,8-Cineole | C10H18O | 1031 | 51.64 | 0.80 | RI, MS, Co |
| γ-Terpinene | C10H16 | 1057 | 0.67 | 0.19 | RI, MS, Co |
| Isoterpinolene | C10H16 | 1085 | 0.23 | 0.13 | RI, MS |
| Linalool | C10H18O | 1099 | 0.28 | 0.06 | RI, MS |
| Camphor | C10H18O | 1145 | 1.84 | 0.05 | RI, MS, Co |
| Camphene hydrate | C10H18O | 1152 | 0.16 | - | RI, MS |
| Borneol | C10H18O | 1159 | 0.38 | - | RI, MS |
| Benzenepropanal | C9H10O | 1167 | - | 7.42 | RI, MS |
| Terpinen-4-ol | C10H18O | 1177 | 1.4 | - | RI, MS, Co |
| α-Terpineol | C10H18O | 1191 | 9.85 | 0.68 | RI, MS, Co |
| Benzylacetone | C10H12O | 1211 | 0.53 | 26.77 | RI, MS |
| Fenchyl acetate | C12H20O2 | 1218 | 0.55 | - | RI, MS |
| Nonanoic acid | C9H18O2 | 1283 | - | 0.35 | RI, MS |
| α-Cubebene | C15H24 | 1352 | 0.50 | - | RI, MS |
| α-Terpinyl acetate | C12H20O2 | 1360 | - | 0.24 | RI, MS |
| α-Copaene | C15H24 | 1372 | 0.45 | - | RI, MS |
| Isoledene | C15H24 | 1375 | 0.38 | - | RI, MS |
| β-Elemene | C15H24 | 1388 | 0.37 | - | RI, MS |
| α-Bergamotene | C15H24 | 1410 | - | 0.56 | RI, MS |
| α-Santalol | C15H24O | 1417 | - | 0.34 | RI, MS |
| β-Caryophyllene | C15H24 | 1420 | 0.44 | 0.07 | RI, MS |
| Undecanoic acid | C11H22O2 | 1441 | - | 0.27 | RI, MS |
| α-Humulene | C15H24 | 1455 | 0.13 | - | RI, MS, Co |
| Alloaromadendrene | C15H24 | 1463 | - | 0.12 | RI, MS |
| β-Patchoulene | C15H24 | 1465 | 0.41 | - | RI, MS |
| Germacrene D | C15H24 | 1480 | 1.13 | - | RI, MS |
| β-Selinene | C15H24 | 1485 | 0.14 | 0.05 | RI, MS |
| Valencene | C15H24 | 1489 | - | 0.09 | RI, MS |
| α-Selinene | C15H24 | 1492 | 1.62 | 0.05 | RI, MS |
| α-Muurolene | C15H24 | 1497 | 0.34 | - | RI, MS |
| Zingiberene | C15H24 | 1498 | 1.05 | - | RI, MS, Co |
| Calamenene | C15H22 | 1504 | 0.42 | - | RI, MS |
| δ-Cadinene isomers | C15H24 | 1523 | 5.44 | 0.42 | RI, MS |
| Guaiacylacetone | C10H12O3 | 1528 | - | 10.13 | RI, MS |
| Viridiflorol | C15H26O | 1588 | - | 1.42 | RI, MS |
| τ-Muurolol | C15H26O | 1643 | - | 0.04 | RI, MS |
| β-Eudesmol | C15H26O | 1648 | - | 0.03 | RI, MS |
| α-Cadinol | C15H26O | 1654 | 0.65 | - | RI, MS |
| Z-α-trans-Bergamotol | C15H24O | 1685 | - | 0.18 | RI, MS |
| Aristolone | C15H22O | 1765 | - | 0.05 | RI, MS |
| 1,7-Diphenyl-5-hydroxy-3-heptanone | C19H20O | 1785 | - | 17.68 | RI, MS |
| 3-Phenylbutanol | C10H14O | 1789 | - | 0.35 | RI, MS |
| Monoterpenoids | 81.84 | 9.69 | |||
| Sesquiterpenoids | 13.47 | 3.42 | |||
| Total | 96.39 | 68.90 | |||
| Samples | LD50 (μg/Adult) | 95% Fiducial Limits | Slope ± SE | Chisquare (χ2) |
|---|---|---|---|---|
| Essential Oil | 20.71 | 2.96–35.85 | 1.39 ± 0.41 | 14.22 |
| SEF-sample | 82.72 | 62.28–100.29 | 1.49 ± 0.28 | 10.13 |
| Pyrethrins * | 0.26 | 0.22–0.30 | 3.34 ± 0.32 | 13.11 |
| Compounds | Feeding Deterrent Indices (%) (Mean ± SD) | ||||
|---|---|---|---|---|---|
| Concentration * (ppm) | |||||
| 15 | 50 | 150 | 500 | 1500 | |
| 1 | 14.56 ± 1.12 | 15.16 ± 1.05 | 16.24 ± 0.68 | 16.50 ± 3.15 | 18.21 ± 2.71 |
| 2 | 1.13 ± 0.98 | 6.29 ± 1.25 | 9.33 ± 0.88 | 18.09 ± 1.59 | 18.94 ± 1.38 |
| 3 | 12.78 ± 1.30 | 13.91 ± 1.81 | 19.12 ± 2.80 | 19.68 ± 2.75 | 19.79 ± 2.62 |
| 4 | 15.98 ± 2.20 | 18.10 ± 1.39 | 19.72 ± 0.75 | 23.79 ± 2.23 | 26.99 ± 1.27 |
| 5 | 12.84 ± 2.79 | 13.67 ± 0.82 | 14.48 ± 1.07 | 15.68 ± 1.51 | 20.34 ± 0.78 |
| 6 | 10.38 ± 1.75 | 12.60 ± 1.07 | 16.09 ± 2.18 | 19.94 ± 1.32 | 35.81 ± 2.24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, M.; Guo, S.; Zhang, W.; Geng, Z.; Liang, J.; Du, S.; Deng, Z.; Wang, Y. Chemical Constituents of Supercritical Extracts from Alpinia officinarum and the Feeding Deterrent Activity against Tribolium castaneum. Molecules 2017, 22, 647. https://doi.org/10.3390/molecules22040647
Xin M, Guo S, Zhang W, Geng Z, Liang J, Du S, Deng Z, Wang Y. Chemical Constituents of Supercritical Extracts from Alpinia officinarum and the Feeding Deterrent Activity against Tribolium castaneum. Molecules. 2017; 22(4):647. https://doi.org/10.3390/molecules22040647
Chicago/Turabian StyleXin, Mintong, Shanshan Guo, Wenjuan Zhang, Zhufeng Geng, Junyu Liang, Shushan Du, Zhiwei Deng, and Yongyan Wang. 2017. "Chemical Constituents of Supercritical Extracts from Alpinia officinarum and the Feeding Deterrent Activity against Tribolium castaneum" Molecules 22, no. 4: 647. https://doi.org/10.3390/molecules22040647






