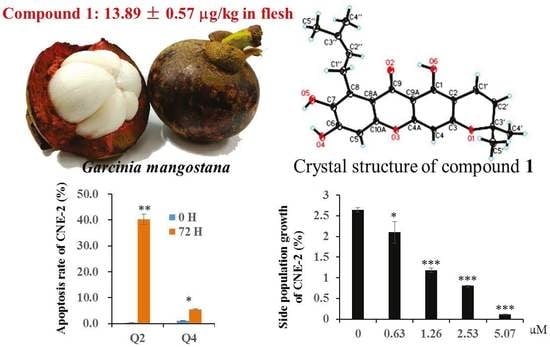
Xanthones from the Pericarp of Garcinia mangostana
Abstract
:1. Introduction
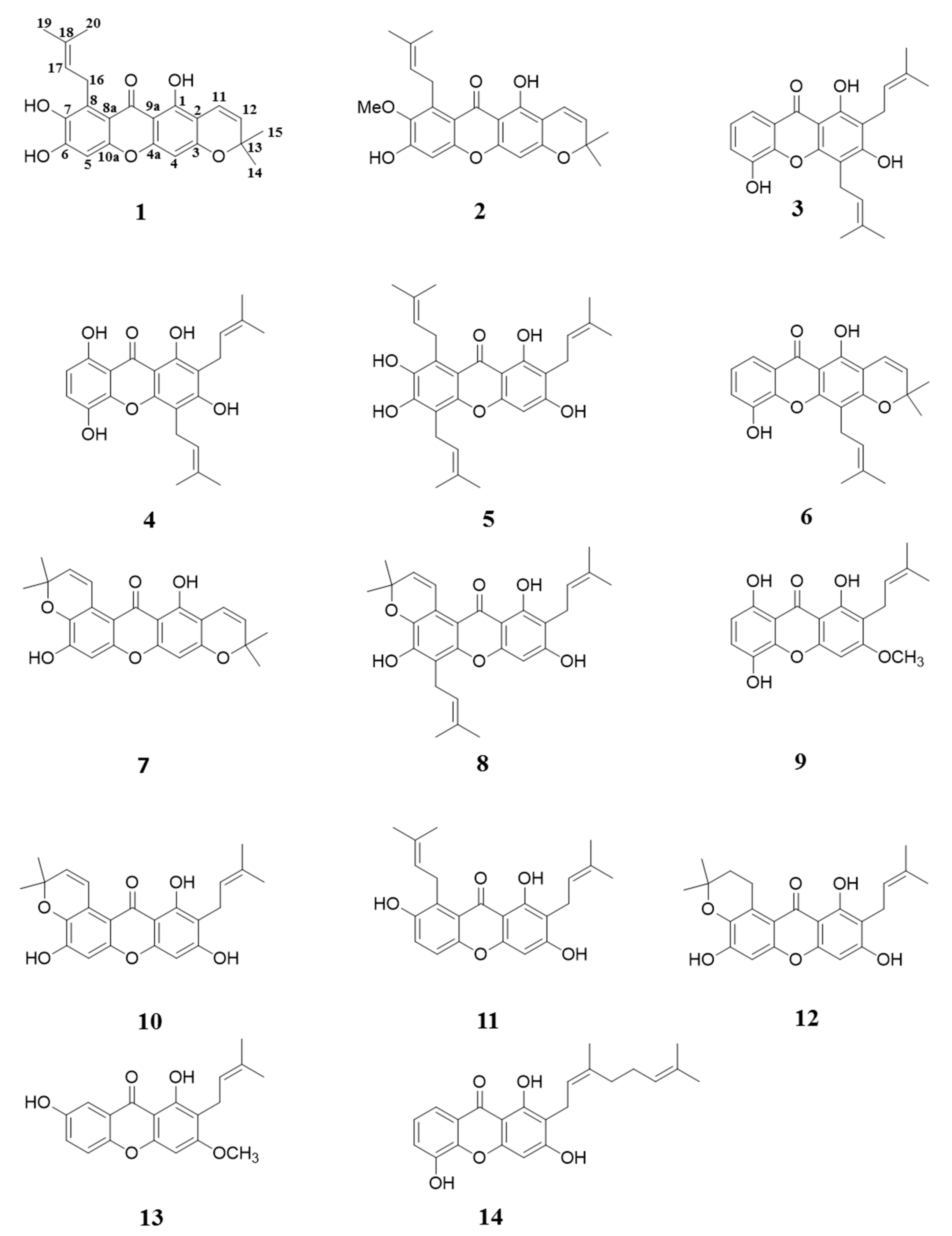
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Sample Preparation and Isolation
3.4. Cell Culture
3.5. MTT Assay
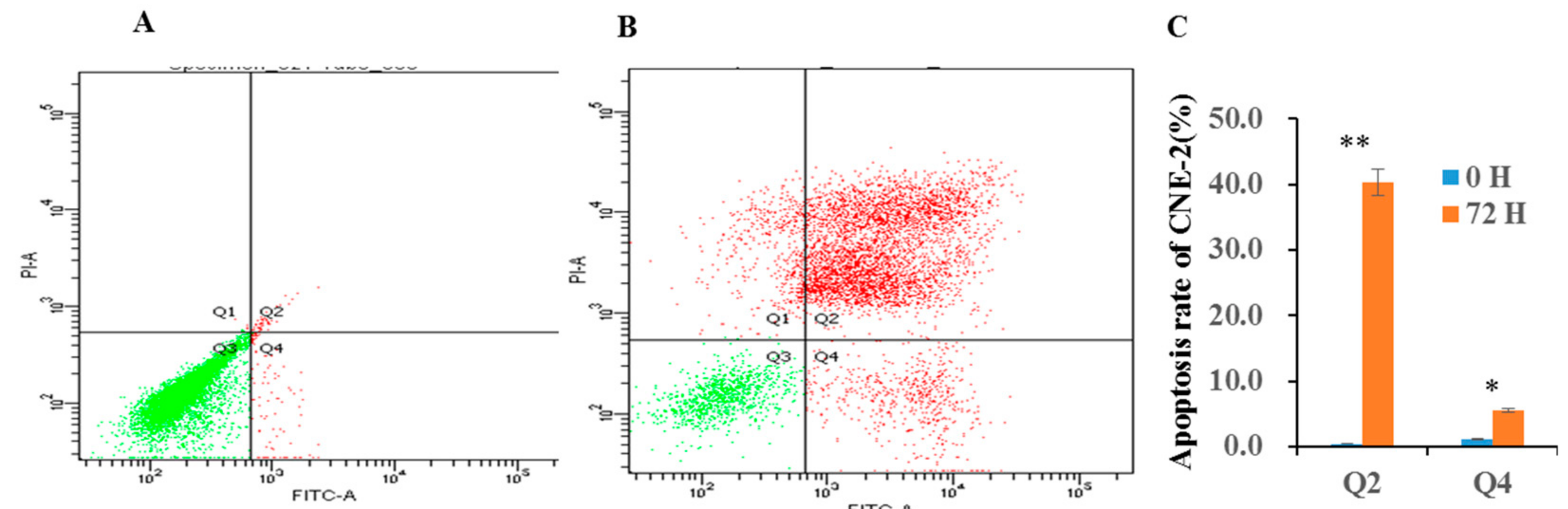
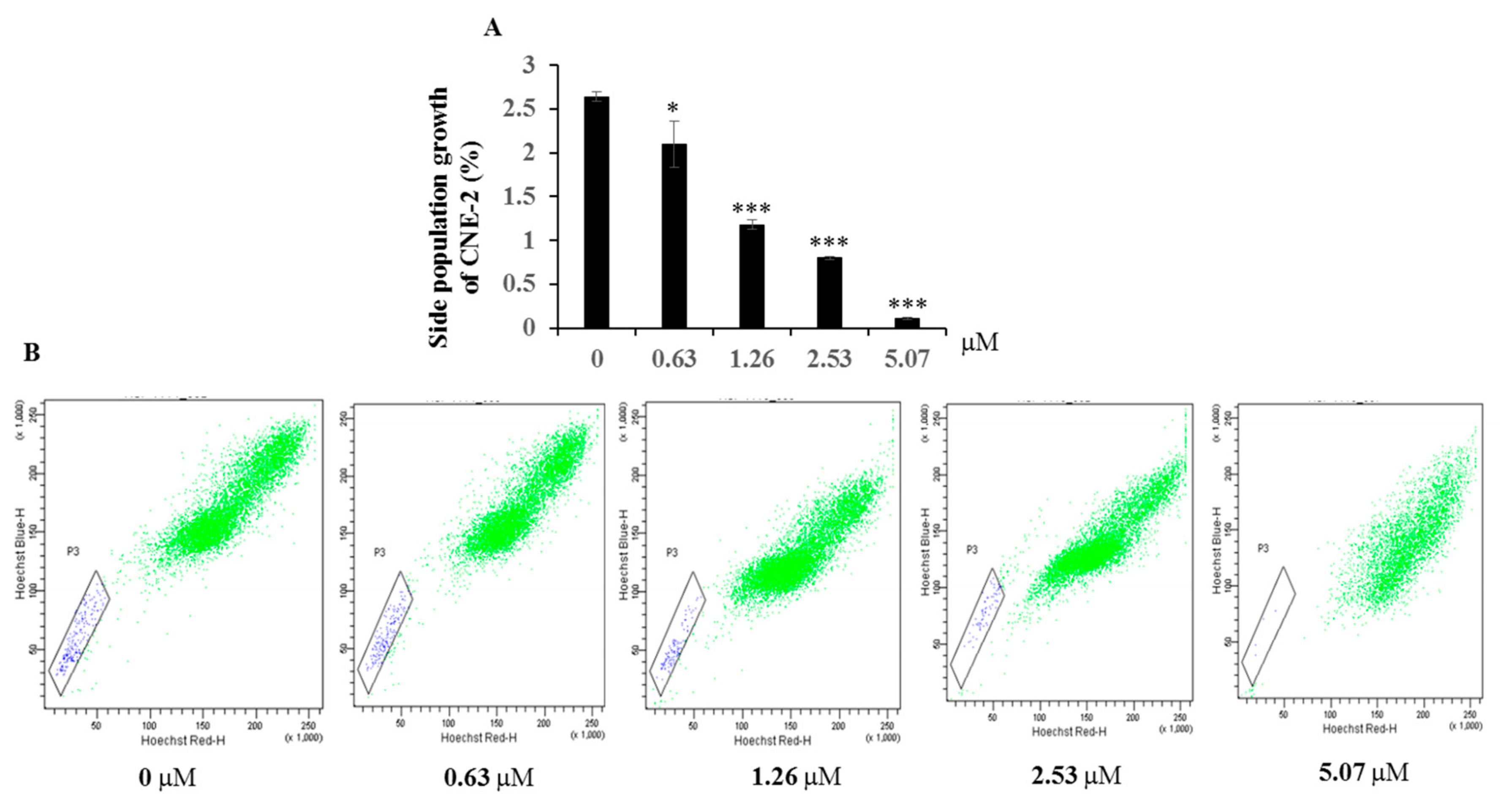
3.6. FCM Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, Y.; Li, D.; Jia, C.; Xue, C.; Bai, J.; Li, Z.; Hua, H. Three new xanthones from the leaves of Garcinia lancilimba. J. Nat. Med. 2015, 70, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.; Shaaban, M.I.; Ross, S.A. Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana. Fitoterapia 2014, 98, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, S.; Tang, C.; Li, J.; Yang, G. Caged polyprenylated xanthones from the resin of Garcinia hanburyi. Fitoterapia 2015, 109, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Thoison, O.; Fahy, J.; Dumontet, V.; Chiaroni, A.; Riche, C.; Tri, M.V.; Sevenet, T. Cytotoxic prenylxanthones from Garcinia bracteata. J. Nat. Prod. 2000, 63, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sriyatep, T.; Siridechakorn, I.; Maneerat, W.; Pansanit, A.; Ritthiwigrom, T.; Andersen, R.J.; Laphookhieo, S. Bioactive prenylated xanthones from the young fruits and flowers of Garcinia cowa. J. Nat. Prod. 2015, 78, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, S.; Komutiban, O.; Ratananukul, P.; Chimnoi, N.; Lartpornmatulee, N.; Suksamrarn, A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem. Pharm. Bull. 2006, 54, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Siridechakorn, I.; Phakhodee, W.; Ritthiwigrom, T.; Promgool, T.; Deachathai, S.; Cheenpracha, S.; Prawat, U.; Laphookhieo, S. Antibacterial dihydrobenzopyran and xanthone derivatives from Garcinia cowa stem barks. Fitoterapia 2012, 83, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tsai, M.L.; Chiou, L.Y.; Ho, C.T.; Pan, M.H. Antitumor Activity of Garcinol in Human Prostate Cancer Cells and Xenograft Mice. J. Agric. Food Chem. 2015, 63, 9047–9052. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, K.; Yamakuni, T.; Kondo, N.; Arakawa, T.; Oosawa, K.; Shimura, S.; Inoue, H.; Ohizumi, Y. γ-Mangostin inhibits IκB kinase activity and decreases lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol. Pharmacol. 2004, 66, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.H.; Kim, I.R.; Kim, H.J.; Park, B.S.; Yu, S.B. α-Mangostin Induces Apoptosis and Cell Cycle Arrest in Oral Squamous Cell Carcinoma Cell. Evid.-Based Complement. Altern. Med. 2016, 2016, 5352412. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, Y.; Zhong, Y.; Cen, J.; Wang, L.; Liu, Y.; Zhu, Y.; Tong, L.; Wei, Q. Amelioration of Experimental Autoimmune Encephalomyelitis by Isogarcinol Extracted from Garcinia mangostana L. Mangosteen. J. Agric. Food Chem. 2016, 64, 9012–9021. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Ninomiya, K.; Tagashira, Y.; Maejima, K.; Yoshida, T.; Amakura, Y. Polyphenolic Constituents of the Pericarp of Mangosteen (Garcinia mangostana L.). J. Agric. Food Chem. 2015, 63, 7670–7674. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhou, H.; Wang, M.; Cen, J.; Wei, Q. Immune regulation and anti-inflammatory effects of isogarcinol extracted from Garcinia mangostana L. against collagen-induced arthritis. J. Agric. Food Chem. 2014, 62, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.C.; Hsu, Y.L.; Liu, C.K.; Kuo, P.L. α-Mangostin, a dietary xanthone, induces autophagic cell death by activating the AMP-activated protein kinase pathway in glioblastoma cells. J. Agric. Food Chem. 2011, 59, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Thida, M.; Kim, D.W.; Tran, T.T.; Pham, M.Q.; Lee, H.; Kim, I.; Lee, J.W. Gambogic acid induces apoptotic cell death in T98G glioma cells. Bioorg. Med. Chem. Lett. 2016, 26, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Ee, G.C.; See, I.; Teh, S.S.; Daud, S. A new furanoxanthone from Garcinia mangostana. J. Asian Nat. Prod. Res. 2014, 16, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Di Francia, R.; Rinaldi, L.; Cillo, M.; Varriale, E.; Facchini, G.; D’Aniello, C.; Marotta, G.; Berretta, M. Antioxidant diet and genotyping as tools for the prevention of liver disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5155–5163. [Google Scholar] [PubMed]
- Grundy, A.; Poirier, A.E.; Khandwala, F.; McFadden, A.; Friedenreich, C.M.; Brenner, D.R. Cancer incidence attributable to insufficient fruit and vegetable consumption in Alberta in 2012. CMAJ Open 2016, 4, E760–E767. [Google Scholar] [CrossRef] [PubMed]
- Zelefack, F.; Guilet, D.; Fabre, N.; Bayet, C.; Chevalley, S.; Ngouela, S.; Lenta, B.N.; Valentin, A.; Tsamo, E.; Dijoux-Franca, M.G. Cytotoxic and antiplasmodial xanthones from Pentadesma butyracea. J. Nat. Prod. 2009, 72, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-H.D.; Vo, H.T.; Pham, H.D.; Connolly, J.D.; Harrison, L.J. Xanthones from the bark of Garcinia merguensis. Phytochemistry 2003, 63, 467–470. [Google Scholar] [CrossRef]
- Bennett, G.J.; Lee, H.H.; Lee, A.P. Synthesis of minor xanthones from Garcinia mangostana. J. Nat. Prod. 1990, 53, 1463–1470. [Google Scholar] [CrossRef]
- Sakai, S.-I.; Katsura, M.; Takayama, H.; Aimi, N.; Chokkethaworn, N.; Suttajit, M. The Structure of Garcinone E. Chem. Pharm. Bull. 1993, 41, 958–960. [Google Scholar] [CrossRef]
- Akgul, Y.Y.; Anil, H. Benzofurans and another constituent from seeds of Styrax officinalis. Phytochemistry 2003, 63, 939–943. [Google Scholar] [CrossRef]
- Jung, H.A.; Su, B.N.; Keller, W.J.; Mehta, R.G.; Kinghorn, A.D. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Khan, N.U.-D. Two xanthones from Garcinia mangostana. Phytochemistry 1988, 27, 3694–3696. [Google Scholar] [CrossRef]
- Sen, A.K.; Sarkar, K.K.; Mazumder, P.C.; Banerji, N.; Uusvuori, R.; Hase, T.A. The Structures of Garcinones A, B and C: Three new xanthones from Garcinia mangostana. Phytochemistry 1982, 21, 1747–1750. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Wiriyachitra, P.; Taylor, W.C. Chemical constituents of Garcinia mangostana. J. Nat. Prod. 1987, 50, 474–478. [Google Scholar] [CrossRef]
- Asai, F.; Tosa, H.; Tanaka, T.; Iinuma, M. A xanthone from pericarps of Garcinia mangostana. Phytochemistry 1995, 39, 943–944. [Google Scholar] [CrossRef]
- Nilar; Harrison, L.J. Xanthones from the heartwood of Garcinia mangostana. Phytochemistry 2002, 60, 541–548. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Position | Compound 1 | |
|---|---|---|
| 13C | 1H | |
| 1 | 157.1 | |
| 2 | 103.6 | |
| 3 | 158.4 | |
| 4 | 93.6 | 6.32, s |
| 4a | 155.6 | |
| 5 | 100.1 | 6.76, s |
| 6 | 152.8 | |
| 7 | 141.2 | |
| 8 | 127.6 | |
| 8a | 109.6 | |
| 9 | 181.7 | |
| 9a | 102.9 | |
| 10a | 152.0 | |
| 11 | 114.8 | 6.61, d, 9.9 |
| 12 | 128.0 | 5.73, d, 9.9 |
| 13 | 77.9 | |
| 14 | 27.4 | 1.43, s |
| 15 | 27.4 | 1.43, s |
| 16 | 25.3 | 4.02, d, 6.8 |
| 17 | 123.5 | 5.18, t, 6.8 |
| 18 | 130.2 | |
| 19 | 25.7 | 1.61, s |
| 20 | 18.3 | 1.78, s |
| Concentration (μM) | Cell Lines | |||||||
|---|---|---|---|---|---|---|---|---|
| CNE-1 | CNE-2 | A549 | H460 | PC-3 | SGC-7901 | U87 | PC12 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.40 | 0 | 5.4 | 10.6 | 0 | 7.27 | 1.94 | 0 | / |
| 0.81 | 4.35 | 12.87 | 11.11 | 0 | 10.08 | 3.37 | 14.26 | / |
| 1.58 | 47.42 | 15.96 | 23.81 | 1.15 | 12.27 | 17.98 | 16.25 | / |
| 3.17 | 63.68 | 51.92 | 49.6 | 20.04 | 32.93 | 47.7 | 26.55 | 2.19 |
| 6.34 | 69.4 | 71.24 | 62.54 | 50.72 | 58.68 | 49.17 | 49.15 | 11.33 |
| 12.69 | 78.42 | 80.01 | 63.18 | 58.45 | 64.59 | 51.74 | 68.24 | 4.66 |
| 25.3 | 83.9 | 83.89 | 73.98 | 84.86 | 72.64 | 54.91 | 72.56 | 23.82 |
| 50.7 | / | / | / | / | / | / | / | 40.57 |
| 101.5 | / | / | / | / | / | / | / | 58.21 |
| IC50 | 3.35 | 4.01 | 4.84 | 7.84 | 6.21 | 8.09 | 6.39 | >50 |
| (2.51 μg/mL) | (19.7 μg/mL) | |||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Li, P.; Li, N.; Zhang, Q.; Bai, X.; Wang, L.; Xiao, Y.; Sun, L.; Yang, Q.; Yan, J. Xanthones from the Pericarp of Garcinia mangostana. Molecules 2017, 22, 683. https://doi.org/10.3390/molecules22050683
Yang R, Li P, Li N, Zhang Q, Bai X, Wang L, Xiao Y, Sun L, Yang Q, Yan J. Xanthones from the Pericarp of Garcinia mangostana. Molecules. 2017; 22(5):683. https://doi.org/10.3390/molecules22050683
Chicago/Turabian StyleYang, Renyue, Ping Li, Nana Li, Qian Zhang, Xue Bai, Lishuo Wang, Yiying Xiao, Lirong Sun, Quan Yang, and Jian Yan. 2017. "Xanthones from the Pericarp of Garcinia mangostana" Molecules 22, no. 5: 683. https://doi.org/10.3390/molecules22050683