Roles of Glycoproteins in the Diagnosis and Differential Diagnosis of Chronic and Latent Keshan Disease
Abstract
:1. Introduction
2. Results
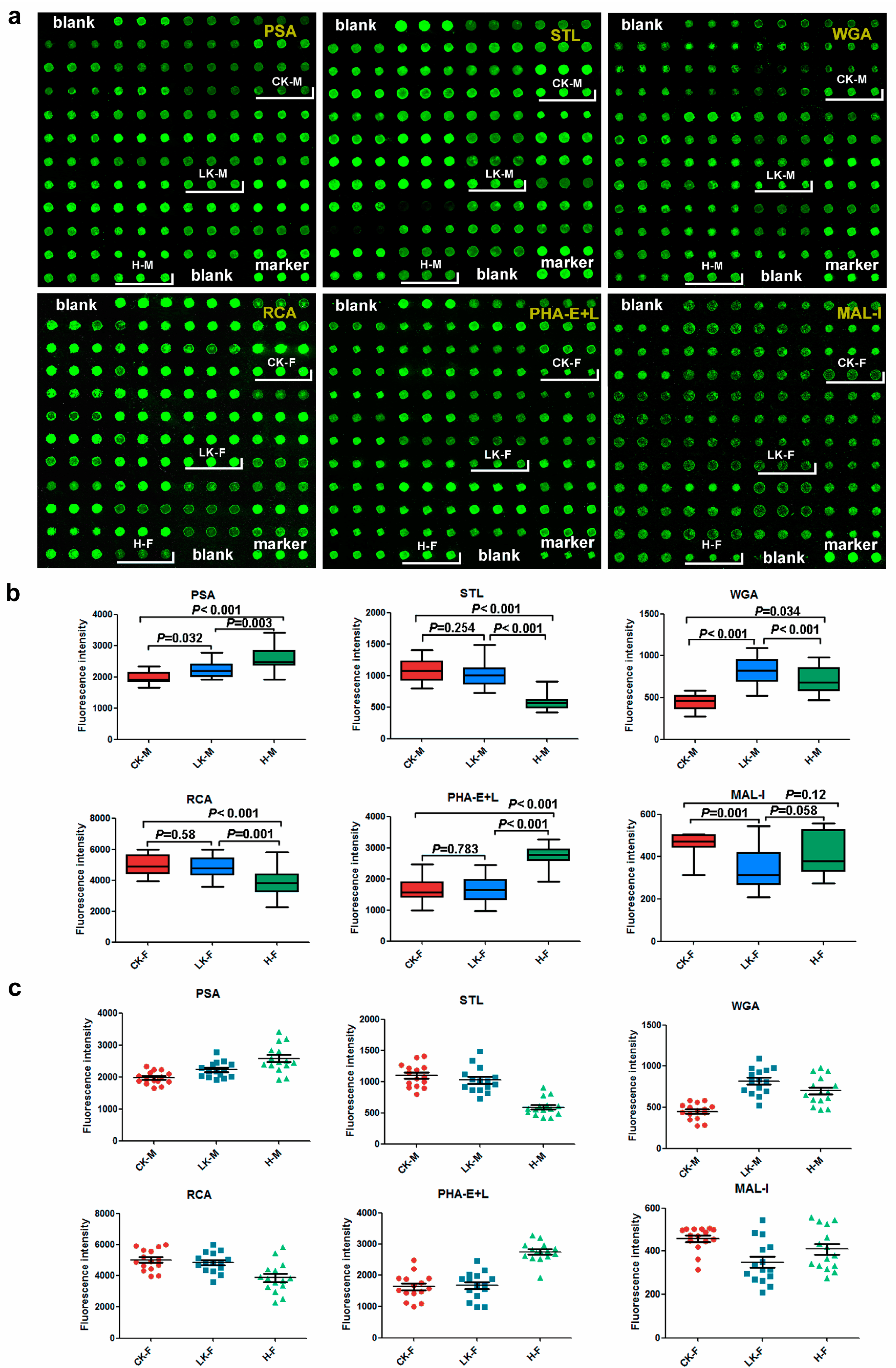
2.1. Male Saliva Lectin Microarray
2.2. Lectin Microarray Analysis of the Female Saliva
2.3. Lectin Microarray of the Male Serum
2.4. Lectin Microarray Analysis of the Female Serum
2.5. Saliva Microarray
2.6. Serum Microarray
3. Discussion
4. Materials and Methods
4.1. The Study Groups
4.2. Peripheral Blood and Whole Saliva Collection
4.3. Saliva and Serum Lectin Microarrays
4.4. Analysis of Saliva and Serum Lectin Microarray
4.5. Saliva and Serum Microarrays
4.6. Statistical Analysis of Saliva and Serum Microarrays
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, Y.; Lai, B.; Niu, X.; Wei, J.; Tan, W.; Wang, X. Long-term prognostic value of major and minor ECG abnormalities in latent Keshan disease with suspect chronic Keshan disease. J. Epidemiol. 2014, 24, 385–391. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Tan, W.; Wang, S.; Wu, C.; Wang, P.; Wang, B.; Su, X.; Zhao, J.; Guo, X.; Xiang, Y. Genome-wide study reveals an important role of spontaneous autoimmunity, cardiomyocyte differentiation defect and anti-angiogenic activities in gender-specific gene expression in Keshan disease. Chin. Med. J. 2014, 127, 72–78. [Google Scholar] [PubMed]
- Lei, C.; Niu, X.; Wei, J.; Zhu, J.; Zhu, Y. Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin. Chim. Acta 2009, 399, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, M.; Hou, J.; Jiang, C.; Li, S.; Wang, T. The prevalence of Keshan disease in China. Int. J. Cardiol. 2013, 168, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, T.; Zhu, M.; Li, L.; Wei, J.; Wu, X.; Guo, M.; Liu, S.; Zhao, H.; Xia, S.; et al. Glycoproteomic analysis of tissues from patients with colon cancer using lectin microarrays and nanoLC-MS/MS. Mol. Biosyst. 2013, 9, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Jian, Q.; Yu, H.; Chen, C.; Li, Z. Establishment of a Lectin Microarray Method for the Rapid Analysis of Glycoprotein and Its Application. Prog. Biochem. Biophys. 2009, 36, 254–259. [Google Scholar] [CrossRef]
- Kautto, L.; Nguyen-Khuong, T.; Everest-Dass, A.; Leong, A.; Zhao, Z.; Willcox, M.D.; Packer, N.H.; Peterson, R. Glycan involvement in the adhesion of Pseudomonas aeruginosa to tears. Exp. Eye Res. 2016, 145, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Sterba, J.; Vancova, M.; Sterbova, J.; Bell-Sakyi, L.; Grubhoffer, L. The majority of sialylated glycoproteins in adult Ixodes ricinus ticks originate in the host, not the tick. Carbohydr. Res. 2014, 389, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pilobello, K.T.; Slawek, D.E.; Mahal, L.K. A ratiometric lectin microarray approach to analysis of the dynamic mammalian glycome. Proc. Natl. Acad. Sci. USA 2007, 104, 11534–11539. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Qin, Y.; Yu, H.; Yu, J.; Wu, H.; Chen, L.; Zhang, P.; Wang, X.; Jia, Z.; Guo, Y.; et al. Avian influenza virus infection risk in humans with chronic diseases. Sci. Rep. 2015, 5, 8971. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ma, T.; Thakur, A.; Yu, H.; Gao, L.; Shi, P.; Li, X.; Ren, H.; Jia, L.; Zhang, S.; et al. Differentially expressed glycosylated patterns of alpha-1-antitrypsin as serum biomarkers for the diagnosis of lung cancer. Glycobiology 2015, 25, 331–400. [Google Scholar] [CrossRef] [PubMed]
- Dassanayaka, S.; Jones, S.P. O-GlcNAc and the cardiovascular system. Pharmacol. Ther. 2014, 142, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Mellor, K.M.; Brimble, M.A.; Delbridge, L.M. Glucose as an agent of post-translational modification in diabetes—New cardiac epigenetic insights. Life Sci. 2015, 129, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Nagy, T.; Champattanachai, V.; Marchase, R.B.; Chatham, J.C. Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-linked N-acetylglucosamine. Am. J. Physiol. Cell Physiol. 2006, 290, C57–C65. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.A.; Powell, P.C.; Dell’italia, L.J.; Chatham, J.C. Cardiac O-GlcNAcylation blunts autophagic signaling in the diabetic heart. Life Sci. 2013, 92, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Medford, H.M.; Porter, K.; Marsh, S.A. Immediate effects of a single exercise bout on protein O-GlcNAcylation and chromatin regulation of cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H114–H123. [Google Scholar] [CrossRef] [PubMed]
- Chun, W.J.; Nah, D.Y.; Bae, J.H.; Chung, J.W.; Lee, H.; Moon, I.S. Glucose-insulin-potassium solution protects ventricular myocytes of neonatal rat in an in vitro coverslip ischemia/reperfusion model. Korean Circ. J. 2015, 45, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Darley-Usmar, V.M.; Ball, L.E.; Chatham, J.C. Protein O-linked beta-N-acetylglucosamine: A novel effector of cardiomyocyte metabolism and function. J. Mol. Cell. Cardiol. 2012, 52, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.T.; Somasuntharam, I.; Gray, W.D.; Shen, M.; Singer, J.M.; Wang, B.; Saafir, T.; Crawford, B.H.; Jiang, R.; Murthy, N.; et al. Bioactive nanoparticles improve calcium handling in failing cardiac myocytes. Nanomedicine 2015, 10, 3343–3357. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; DeMartino, A.M.; Watson, L.J.; Brittian, K.R.; Zafir, A.; Dassanayaka, S.; Hong, K.U.; Jones, S.P. MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression. J. Biol. Chem. 2014, 289, 29665–29676. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tedder, M.E.; Perez, C.E.; Wang, G.; de Jongh Curry, A.L.; To, F.; Elder, S.H.; Williams, L.N.; Simionescu, D.T.; Liao, J. Structural and biomechanical characterizations of porcine myocardial extracellular matrix. J. Mater. Sci. Mater. Med. 2012, 23, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples not available. |
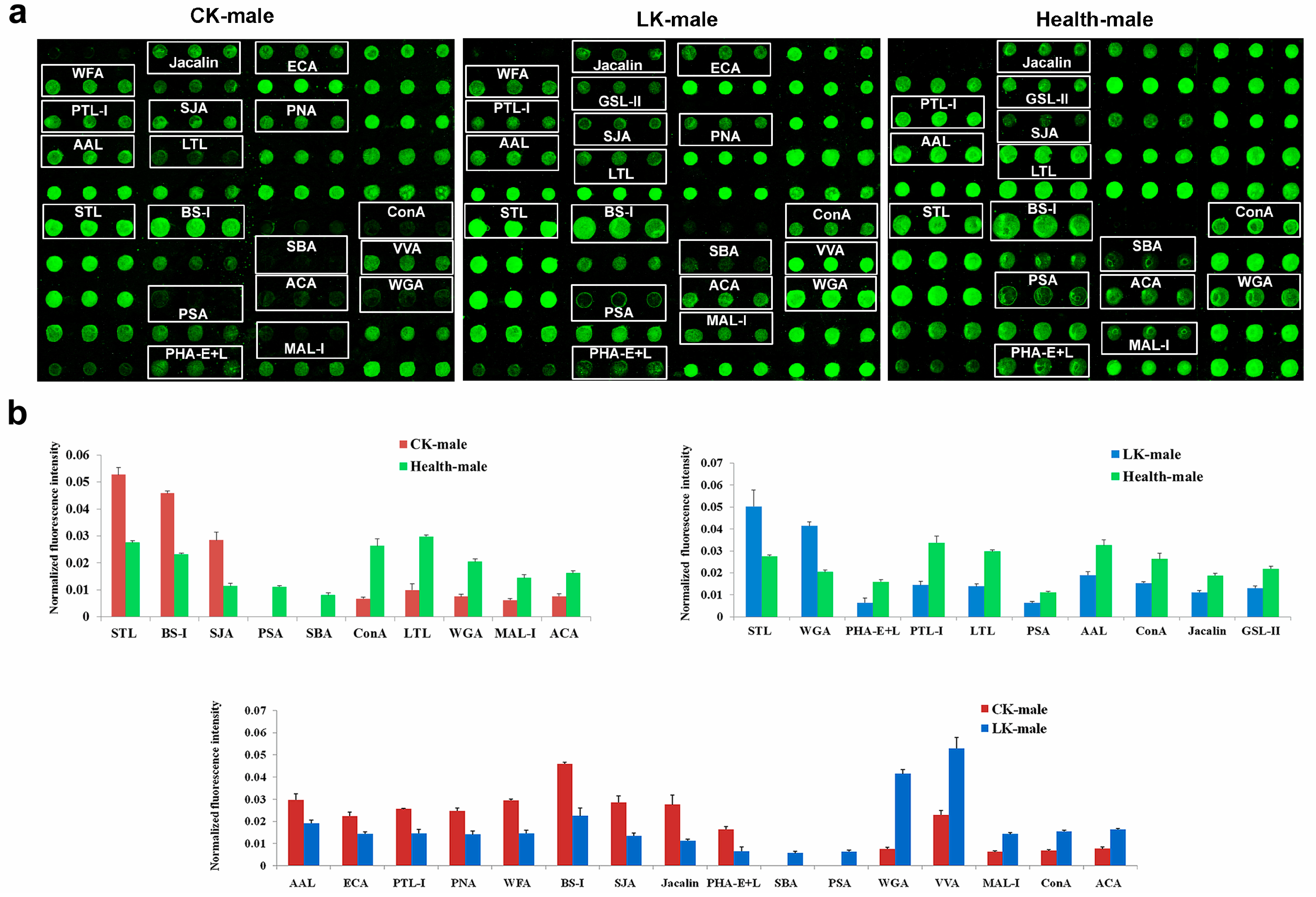
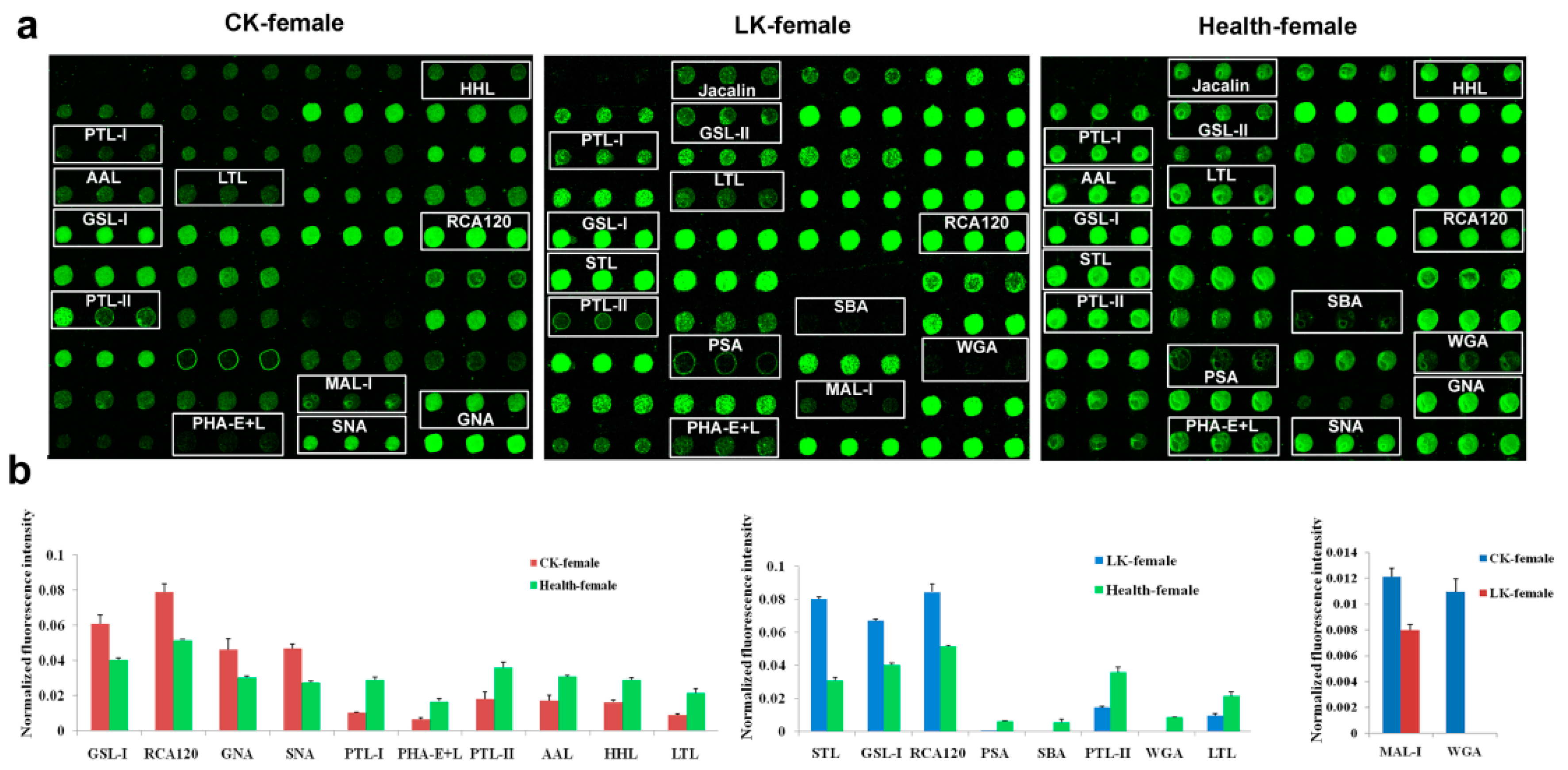
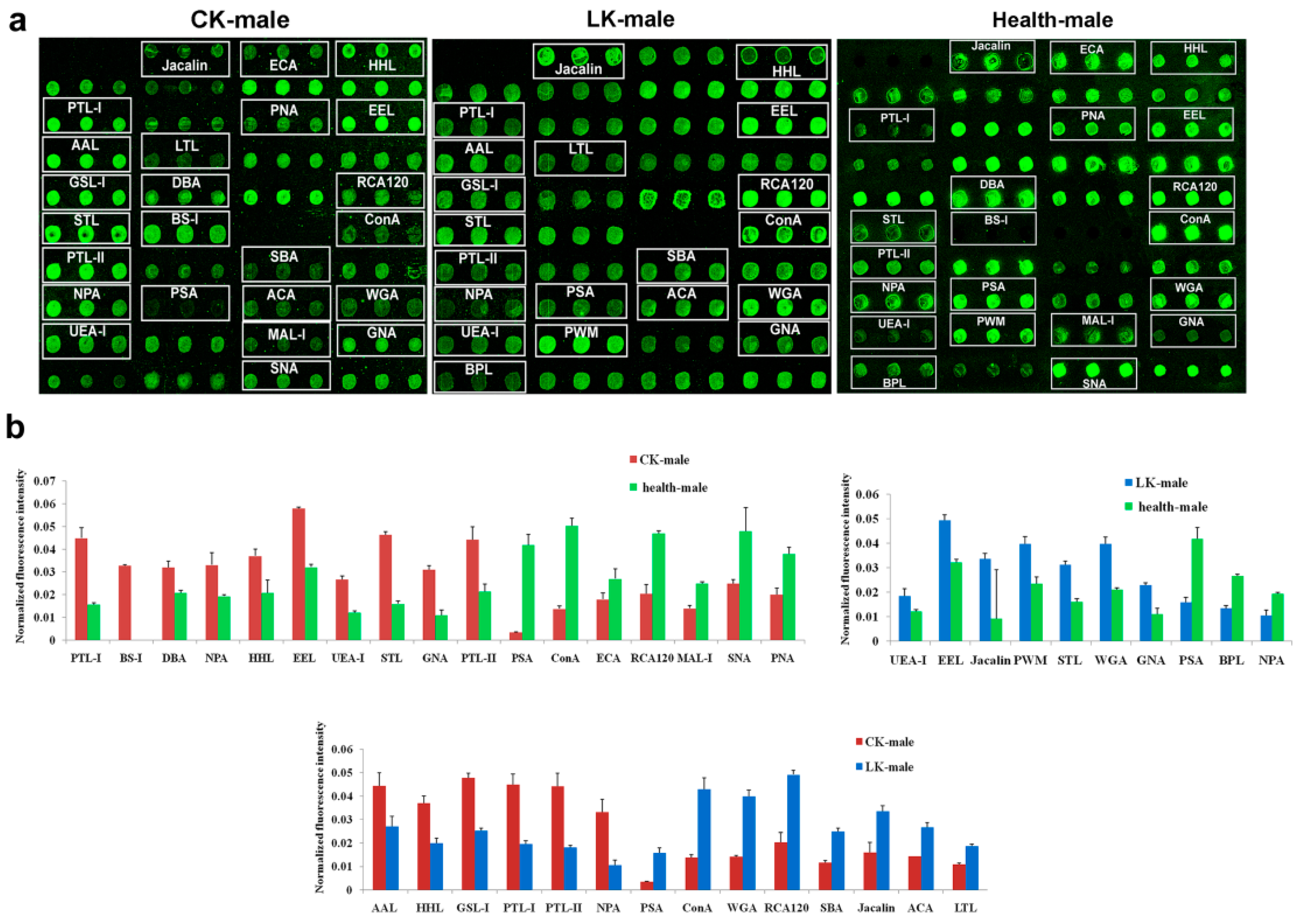
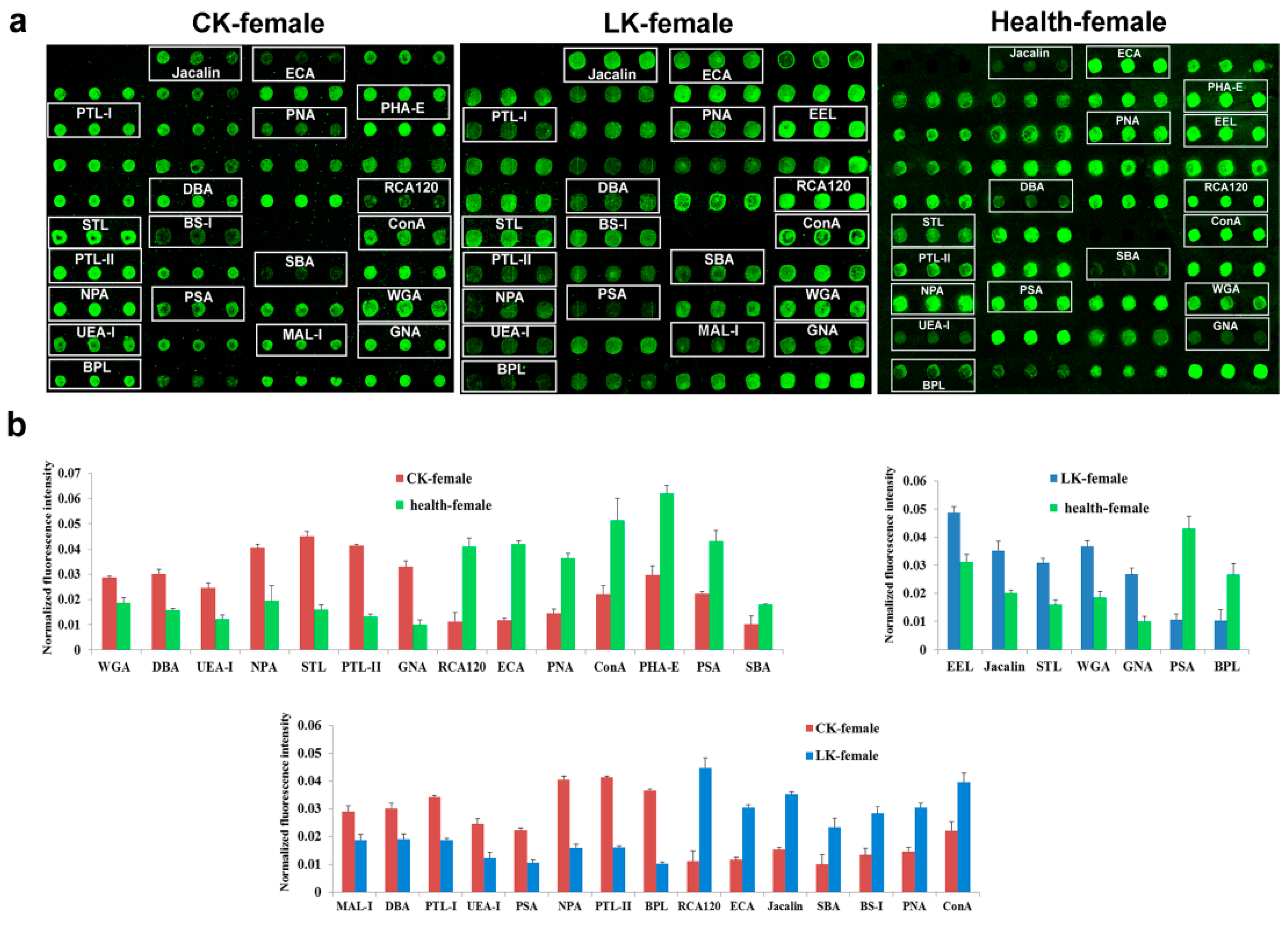

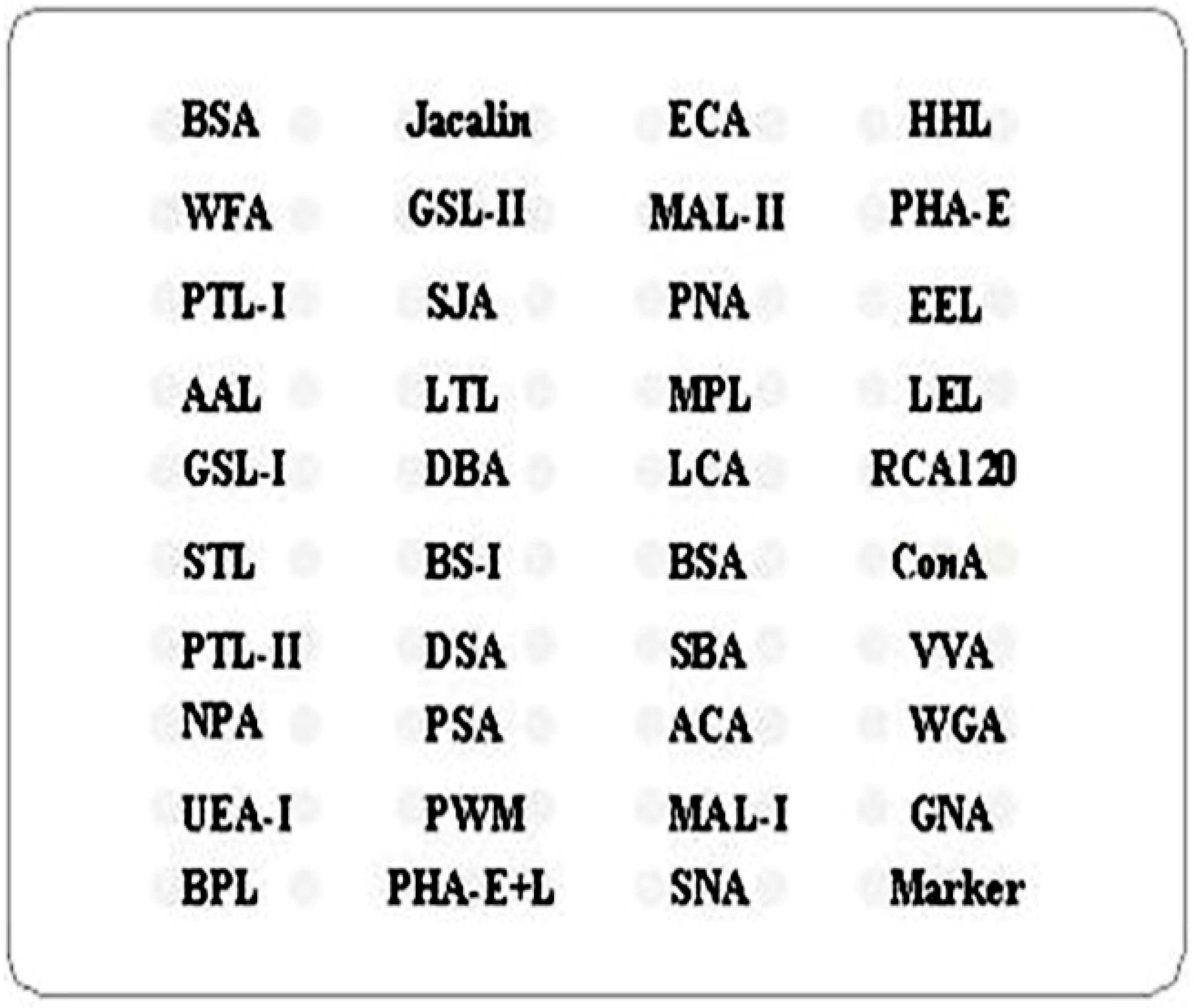
| Lectin | Full Name of the Lectin | Specificity Carbohydrate Chain |
|---|---|---|
| Jacalin | Artocapusintegrifolia | Galβ1-3GalNAcα-Ser/Thr(T), GalNAcα-Ser/Thr(Tn), GlcNAcβ1-3-GalNAcα-Ser/Thr(Core3), sialyl-T(ST). not bind to Core2, Core6, and sialyl-Tn (STn) |
| ECA | Erythrinacristagalli | Galβ-1,4GlcNAc (type II), Galβ1-3GlcNAc (type I) |
| HHL | Hippeastrum Hybrid Lectin | High-Mannose, Manα1-3Man, Manα1-6Man, Man5-GlcNAc2-Asn |
| WFA | Wisteria Floribunda Lectin | terminating in GalNAcα/β1-3/6Gal |
| GSL-II | Griffonia (Bandeiraea) Simplicifolia Lectin II | GlcNAc and agalactosylated tri/tetra antennary glycans |
| MAL-II | Maackia Amurensis Lectin II | Siaα2-3Galβ1-4Glc(NAc)/Glc, Siaα2-3Gal, Siaα2-3, Siaα2-3GalNAc |
| PHA-E | Phaseolus vulgaris Agglutinin (E) | Bisecting GlcNAc, biantennary complex-type N-glycan with outer Gal |
| PTL-I | Psophocarpus Tetragonolobus Lectin I | GalNAc, GalNAcα-1,3Gal, GalNAcα-1,3Galβ-1,3/4Glc |
| SJA | Sophora Japonica Agglutinin | Terminal in GalNAc and Gal, anti-A and anti-B human blood group |
| PNA | Peanut Agglutinin | Galβ1-3GalNAcα-Ser/Thr(T) |
| EEL | Euonymus Europaeus Lectin | Galα1-3(Fucα1-2)Gal(blood group B antigen) |
| AAL | Aleuria Aurantia Lectin | Fucα1-6 GlcNAc(core fucose), Fucα1-3(Galβ1-4)GlcNAc |
| LTL | Lotus Tetragonolobus Lectin | Fucα1-3Galβ1-4GlcNAc, Fucα1-anti-H blood group specificity |
| MPL | Maclura Pomifera Lectin | Galβ1-3GalNAc, GalNAc |
| LEL | Lycopersicon Esculentum (Tomato) Lectin | (GlcNAc)n, high mannose-type N-glycans |
| GSL-I | Griffonia (Bandeiraea) Simplicifolia Lectin I | αGalNAc, αGal, anti-A and B |
| DBA | Dolichos Biflorus Agglutinin | αGalNAc, Tn antigen, GalNAcα1-3((Fucα1-2))Gal (blood group A antigen) |
| LCA | Lens Culinaris Agglutinin | α-D-Man, Fucα-1,6GlcNAc, α-D-Glc |
| STL | Solanum Tuberosum (Potato) Lectin | trimers and tetramers of GlcNAc, core (GlcNAc) of N-glycan, oligosaccharide containing GlcNAc and MurNAc |
| PTL-II | Psophocarpus Tetragonolobus Lectin II | Gal, blood group H , T-antigen |
| DSA | Daturastramonium | (GlcNAc) 2-4, polyLacNAc and LacNAc (NA3, NA4) |
| VVA | Vicia Villosa Lectin | terminal GalNAc, GalNAcα-Ser/Thr(Tn), GalNAcα1-3Gal |
| MAL-I | Maackia Amurensis Lectin I | Galβ-1,4GlcNAc |
| GNA | Galanthusnivalis | High-Mannose, Manα1-3Man |
| NPA | Narcissus Pseudonarcissus Lectin | High-Mannose,Manα1-6Man |
| ACA | Amaranthuscaudatus | Galβ1-3GalNAcα-Ser/Thr (T antigen), sialyl-T(ST) tissue staining patterns are markedly different than those obtained with either PNA or Jacalin |
| BPL | Bauhinia Purpurea Lectin | Galβ1-3GalNAc, Terminal GalNAc |
| PHA-E+L | Phaseolus vulgaris Agglutinin (E+L) | Bisecting GlcNAc, bi-antennary N-glycans, tri- and tetra-antennary complex-type N-glycan |
| SNA | Sambucus Nigra Lectin | Sia2-6Gal/GalNAc |
| RCA120 | Ricinus Communis Agglutinin I | β-Gal, Galβ-1,4GlcNAc (type II), Galβ1-3GlcNAc (type I) |
| BS-I | Bandeiraeasimplicifolia | α-Gal, α-GalNAc, Galα-1,3Gal, Galα-1,6Glc |
| PSA | Pisum Sativum Agglutinin | Fucα-1,6GlcNAc,α-D-Man, α-D-Glc |
| SBA | Soybean Agglutinin | α- or β-linked terminal GalNAc, (GalNAc)n, GalNAcα1-3Gal, blood-group A |
| WGA | Triticum vulgaris | Multivalent Sia and (GlcNAc)n |
| UEA-I | Ulex Europaeus Agglutinin I | Fucα1-2Galβ1-4Glc(NAc) |
| PWM | Phytolaccaamericana | (GlcNAc)n and polyLacNAc |
| ConA | Canavaliaensiformis | High-Mannose, Manα1-6(Manα1-3)Man, αMannose, αGlc |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Fan, Z.; Zhou, B.; Wang, Y.; Du, P.; Tan, W.; Lammi, M.J.; Guo, X. Roles of Glycoproteins in the Diagnosis and Differential Diagnosis of Chronic and Latent Keshan Disease. Molecules 2017, 22, 746. https://doi.org/10.3390/molecules22050746
Wang S, Fan Z, Zhou B, Wang Y, Du P, Tan W, Lammi MJ, Guo X. Roles of Glycoproteins in the Diagnosis and Differential Diagnosis of Chronic and Latent Keshan Disease. Molecules. 2017; 22(5):746. https://doi.org/10.3390/molecules22050746
Chicago/Turabian StyleWang, Sen, Zheng Fan, Bing Zhou, Yingting Wang, Peiru Du, Wuhong Tan, Mikko J. Lammi, and Xiong Guo. 2017. "Roles of Glycoproteins in the Diagnosis and Differential Diagnosis of Chronic and Latent Keshan Disease" Molecules 22, no. 5: 746. https://doi.org/10.3390/molecules22050746





