Transparent Nanotubular TiO2 Photoanodes Grown Directly on FTO Substrates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimizing Electrolyte Composition
2.2. The Growth of TNT Array and Optical Properties
2.3. Crystallinity and Morphology of TNT Arrays
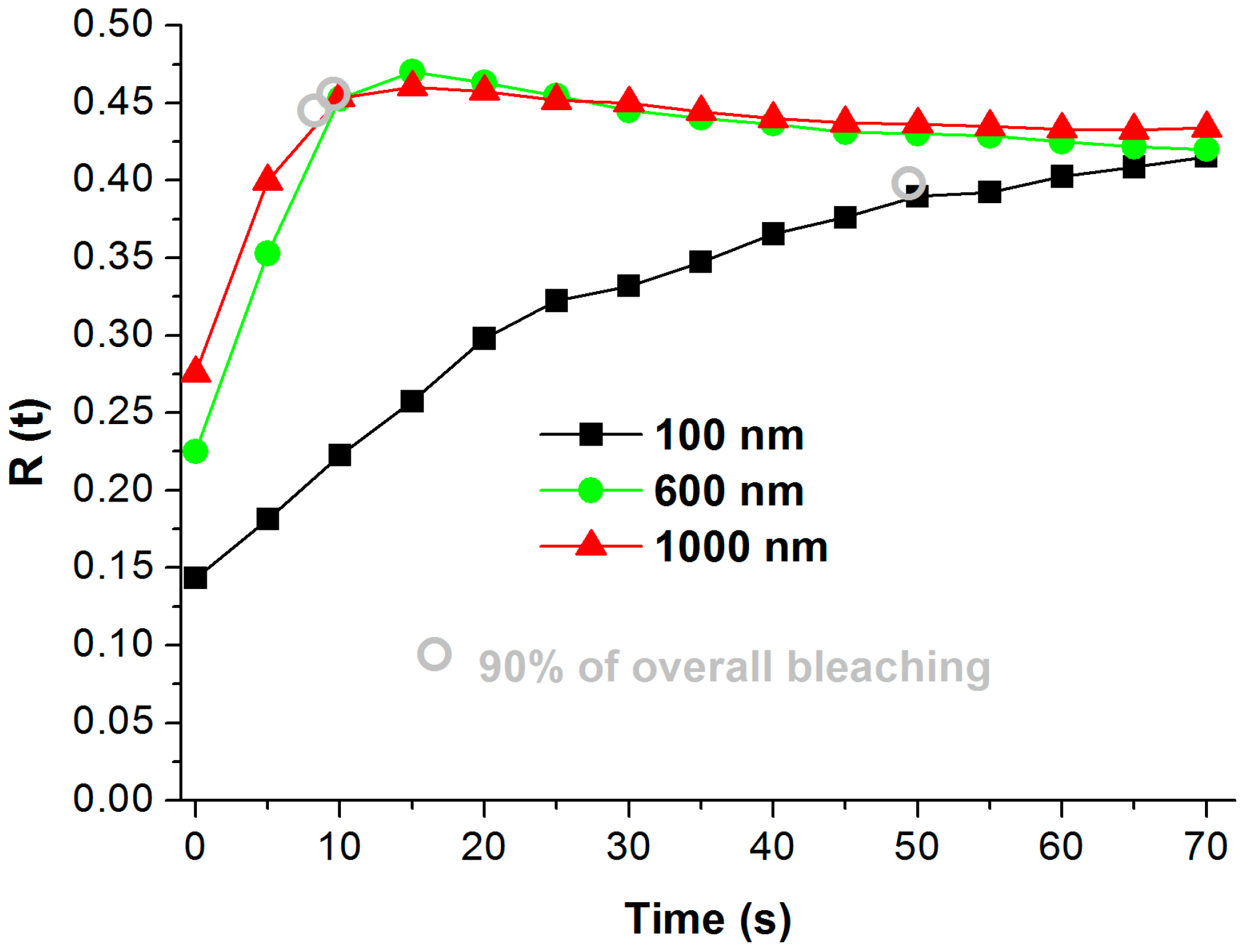
2.4. Photoelectrochemical Activity
2.5. Photocatalytic Activity
3. Experimental Part
3.1. TNT Preparation and Characterization
3.2. Characterization Methods
3.3. Photocatalytic Activity
3.4. Photoelectrochemical Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Grimes, C.A.; Mor, G.K. TiO2 Nanotube Arrays: Synthesis, Properties, and Applications; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Transparent highly ordered TiO2 nanotube arrays via anodization of titanium thin films. Adv. Funct. Mater. 2005, 15, 1291–1296. [Google Scholar] [CrossRef]
- Kathirvel, S.; Su, C.; Yang, C.-Y.; Shiao, Y.-J.; Chen, B.-R.; Li, W.-R. The growth of TiO2 nanotubes from sputter-deposited ti film on transparent conducting glass for photovoltaic applications. Vacuum 2015, 118, 17–25. [Google Scholar] [CrossRef]
- Albu, S.P.; Schmuki, P. Influence of anodization parameters on the expansion factor of TiO2 nanotubes. Electrochim. Acta 2013, 91, 90–95. [Google Scholar] [CrossRef]
- Szkoda, M.; Lisowska-Oleksiak, A.; Grochowska, K.; Skowroński, Ł.; Karczewski, J.; Siuzdak, K. Semi-transparent ordered TiO2 nanostructures prepared by anodization of titanium thin films deposited onto the fto substrate. Appl. Surf. Sci. 2016, 381, 36–41. [Google Scholar] [CrossRef]
- Hoyer, P. Formation of a titanium dioxide nanotube array. Langmuir 1996, 12, 1411–1413. [Google Scholar] [CrossRef]
- Jinsoo, K.; Jonghyun, K.; Myeongkyu, L. Laser welding of nanoparticulate TiO2 and transparent conducting oxide electrodes for highly efficient dye-sensitized solar cell. Nanotechnology 2010, 21, 345203. [Google Scholar]
- Lin, C.-J.; Yu, W.-Y.; Chien, S.-H. Transparent electrodes of ordered opened-end TiO2-nanotube arrays for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2010, 20, 1073–1077. [Google Scholar] [CrossRef]
- Kim, J.Y.; Noh, J.H.; Zhu, K.; Halverson, A.F.; Neale, N.R.; Park, S.; Hong, K.S.; Frank, A.J. General strategy for fabricating transparent TiO2 nanotube arrays for dye-sensitized photoelectrodes: Illumination geometry and transport properties. ACS Nano 2011, 5, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Krysa, J.; Zlamal, M.; Kment, S.; Brunclikova, M.; Hubicka, Z. TiO2 and Fe2O3 films for photoelectrochemical water splitting. Molecules 2015, 20, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.G.; Santanna, M.A.; Pereira, E.C. Dependence of TiO2 nanotube microstructural and electronic properties on water splitting. J. Power Source 2014, 251, 178–186. [Google Scholar] [CrossRef]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X. Branched TiO2 nanorods for photoelectrochemical hydrogen production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.-X.; Liao, J.-Y.; Zhang, R.; Wang, J.; Su, C.-Y.; Kuang, D.-B. Ordered crystalline TiO2 nanotube arrays on transparent fto glass for efficient dye-sensitized solar cells. J. Phys Chem C 2010, 114, 15228–15233. [Google Scholar] [CrossRef]
- Abdi, F.F.; Firet, N.; Dabirian, A.; van de Krol, R. Spray-deposited co-pi catalyzed bivo4: A low-cost route towards highly efficient photoanodes. MRS Proc. 2012, 1446. [Google Scholar] [CrossRef]
- Chappanda, K.; Smith, Y.; Mohanty, S.; Rieth, L.; Tathireddy, P.; Misra, M. Growth and characterization of tio2 nanotubes from sputtered ti film on si substrate. Nanoscale Res. Lett. 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tao, J.; Zhang, Y.; Wu, T.; Tao, H.; Bao, Z. Preparation and characterization of TiO2 nanotube arrays via anodization of titanium films deposited on fto conducting glass at room temperature. Acta Phys.-Chim. Sin. 2008, 24, 2191–2197. [Google Scholar] [CrossRef]
- Pugliese, D.; Lamberti, A.; Bella, F.; Sacco, A.; Bianco, S.; Tresso, E. TiO2 nanotubes as flexible photoanode for back-illuminated dye-sensitized solar cells with hemi-squaraine organic dye and iodine-free transparent electrolyte. Org. Electron. 2014, 15, 3715–3722. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Liu, Y.; Zhou, B.; Cai, W. A new glass substrate photoelectrocatalytic electrode for efficient visible-light hydrogen production: Cds sensitized TiO2 nanotube arrays. Appl. Catal. B Environ. 2010, 95, 408–413. [Google Scholar] [CrossRef]
- Krumpmann, A.; Dervaux, J.; Derue, L.; Douhéret, O.; Lazzaroni, R.; Snyders, R.; Decroly, A. Influence of a sputtered compact TiO2 layer on the properties of TiO2 nanotube photoanodes for solid-state dsscs. Mate. Des. 2017, 120, 298–306. [Google Scholar] [CrossRef]
- Lim, S.L.; Liu, Y.; Li, J.; Kang, E.-T.; Ong, C.K. Transparent titania nanotubes of micrometer length prepared by anodization of titanium thin film deposited on ito. Appl. Surf. Sci. 2011, 257, 6612–6617. [Google Scholar] [CrossRef]
- Krysa, J.; Lee, K.; Pausova, S.; Kment, S.; Hubicka, Z.; Ctvrtlik, R.; Schmuki, P. Self-organized transparent 1d TiO2 nanotubular photoelectrodes grown by anodization of sputtered and evaporated ti layers: A comparative photoelectrochemical study. Chem. Eng. J. 2017, 308, 745–753. [Google Scholar] [CrossRef]
- Ratnawati; Gunlazuardi, J.; Slamet. Development of titania nanotube arrays: The roles of water content and annealing atmosphere. Mater. Chem. Phys. 2015, 160, 111–118. [Google Scholar]
- Tsui, L.-K.; Homma, T.; Zangari, G. Photocurrent conversion in anodized TiO2 nanotube arrays: Effect of the water content in anodizing solutions. J. Phys. Chem. C 2013, 117, 6979–6989. [Google Scholar] [CrossRef]
- Acevedo-Peña, P.; Lartundo-Rojas, L.; González, I. Effect of water and fluoride content on morphology and barrier layer properties of TiO2 nanotubes grown in ethylene glycol-based electrolytes. J. Solid State Electrochem. 2013, 17, 2939–2947. [Google Scholar] [CrossRef]
- Krýsa, J.; Baudys, M.; Mills, A. Quantum yield measurements for the photocatalytic oxidation of acid orange 7 (ao7) and reduction of 2,6-dichlorindophenol (dcip) on transparent TiO2 films of various thickness. Catal. Today 2015, 240, 132–137. [Google Scholar] [CrossRef]
- Kuzmych, O.; Nonomura, K.; Johansson, E.M.J.; Nyberg, T.; Hagfeldt, A.; Skompska, M. Defect minimization and morphology optimization in TiO2 nanotube thin films, grown on transparent conducting substrate, for dye synthesized solar cell application. Thin Solid Films 2012, 522, 71–78. [Google Scholar] [CrossRef]
- Kang, Q.; Liu, S.; Yang, L.; Cai, Q.; Grimes, C.A. Fabrication of pbs nanoparticle-sensitized TiO2 nanotube arrays and their photoelectrochemical properties. ACS Appl. Mater. Interfaces 2011, 3, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Hepburn, J.; Hazafy, D.; O’Rourke, C.; Krysa, J.; Baudys, M.; Zlamal, M.; Bartkova, H.; Hill, C.E.; Winn, K.R.; et al. A simple, inexpensive method for the rapid testing of the photocatalytic activity of self-cleaning surfaces. J. Photochem. Photobiol. A Chem. 2013, 272, 18–20. [Google Scholar] [CrossRef]
- Mills, A.; Hepburn, J.; Hazafy, D.; O’Rourke, C.; Wells, N.; Krysa, J.; Baudys, M.; Zlamal, M.; Bartkova, H.; Hill, C.E.; et al. Photocatalytic activity indicator inks for probing a wide range of surfaces. J. Photochem. Photobiol. A Chem. 2014, 290, 63–71. [Google Scholar]
- Baudys, M.; Krýsa, J.; Mills, A. Smart inks as photocatalytic activity indicators of self-cleaning paints. Catal. Today 2017, 280, 8–13. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

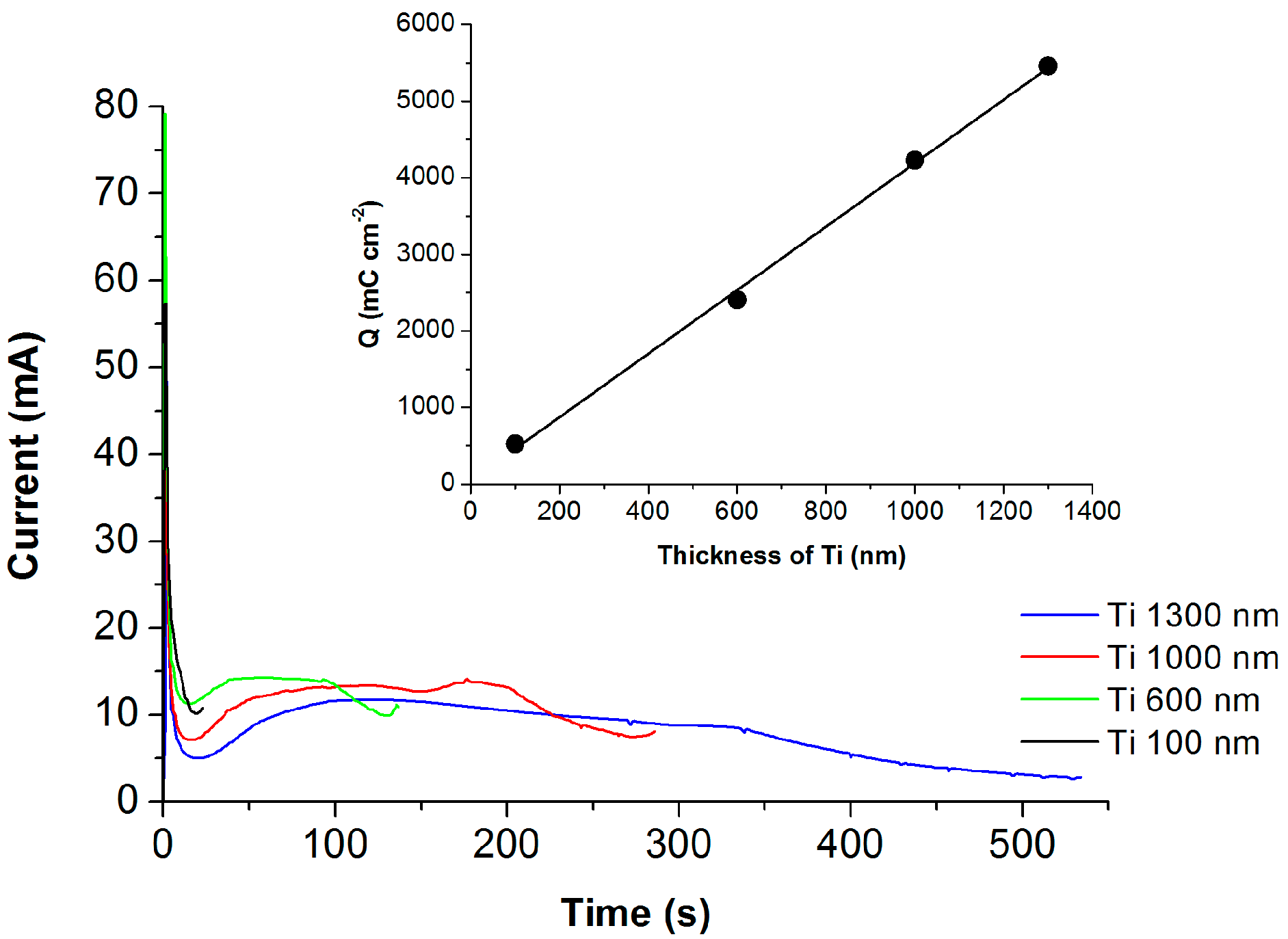
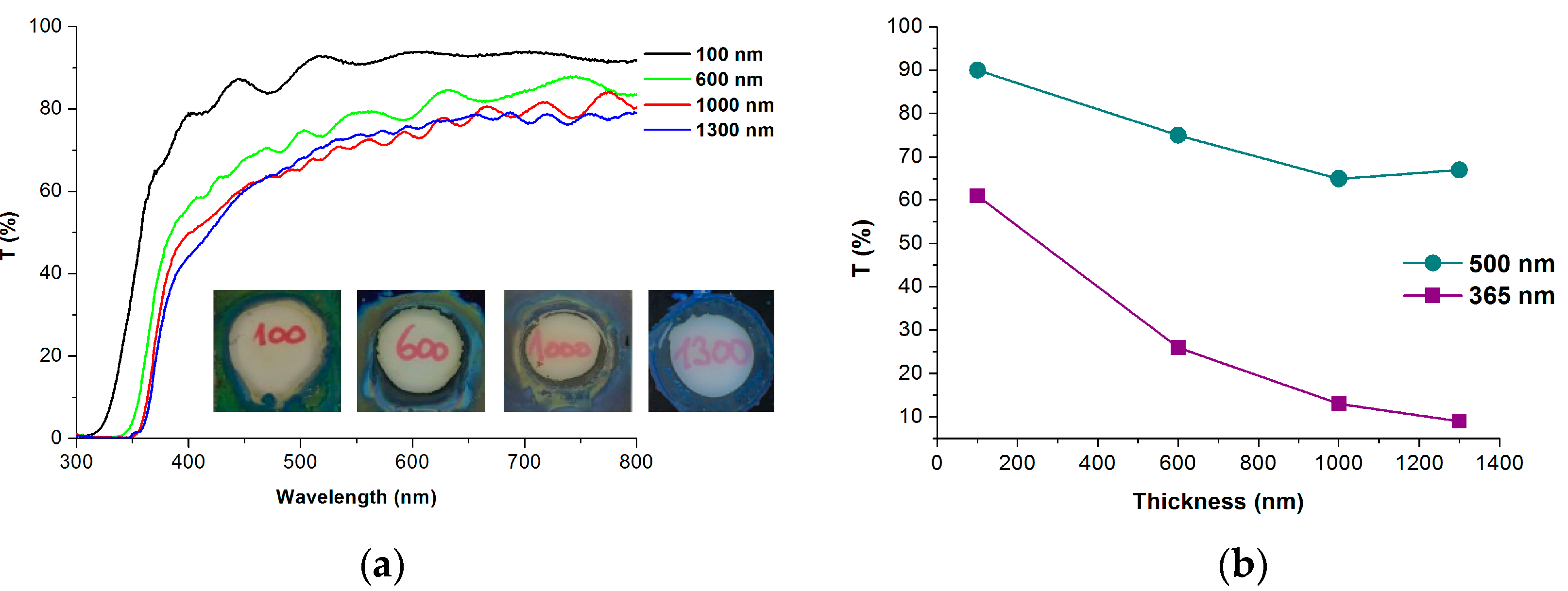

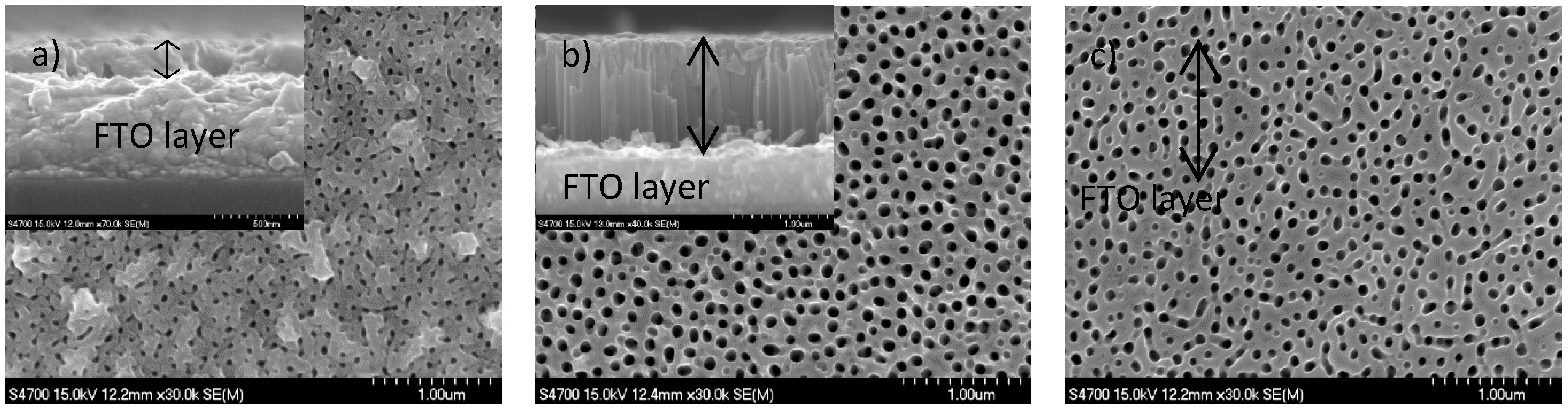
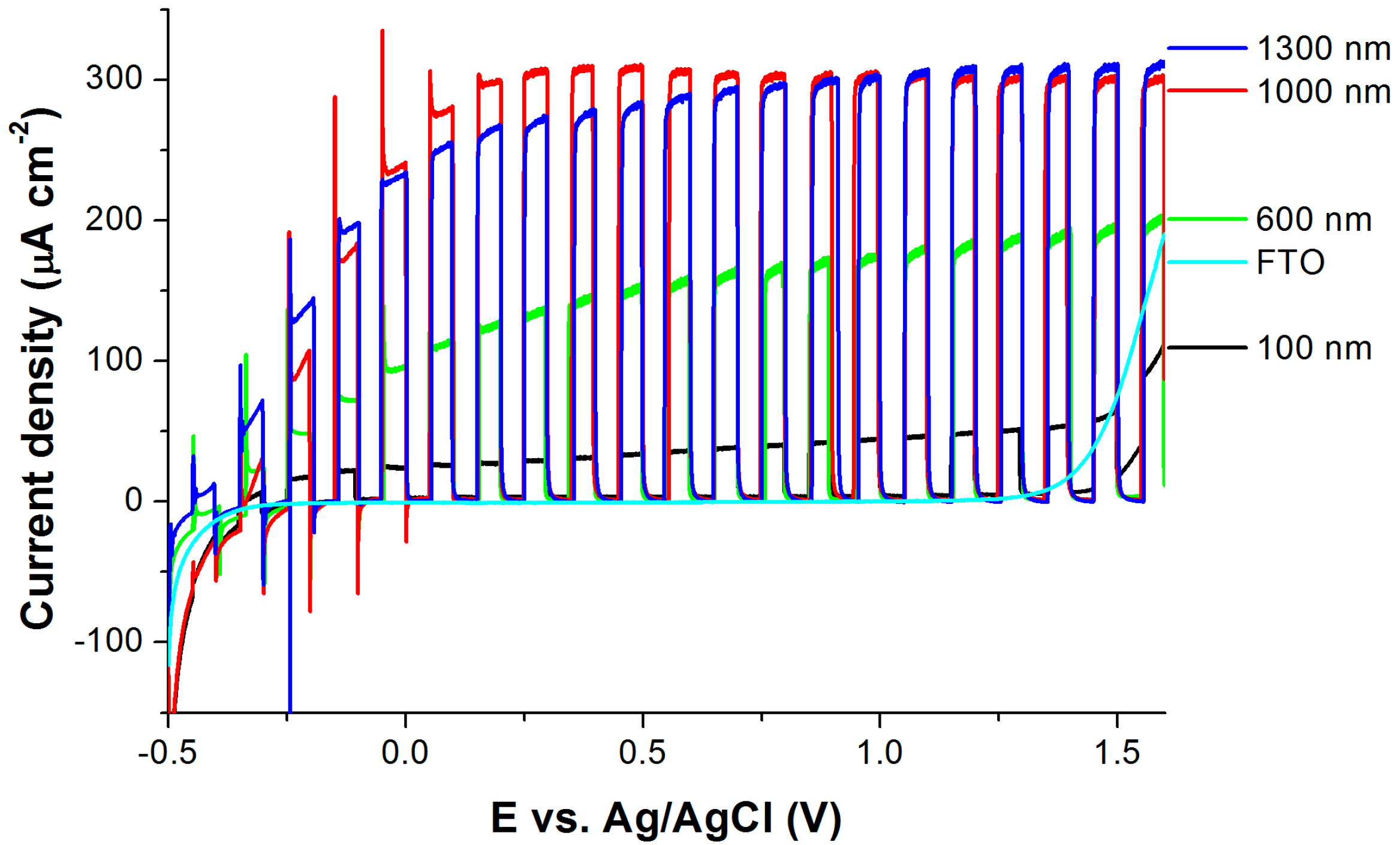
| Name of Sample | Ti Film Thickness (nm) | Anodization Time (s) | Passed Charge (mC cm−2) | TNT Thickness (nm) | Photocurrent Density * (µA cm−2) |
|---|---|---|---|---|---|
| 100 nm | 100 | 25 | 524 | 180 ± 20 | 43 ± 2 |
| 600 nm | 600 | 138 | 2404 | 1000 ± 50 | 196 ± 5 |
| 1000 nm | 1000 | 290 | 4226 | 2450 ± 50 | 300 ± 5 |
| 1300 nm | 1300 | 538 | 5460 | 3500 ± 50 | 294 ± 5 |
| Ti foil | - | 887 | 4155 | - | 315 ± 5 |
| Thickness of Ti on FTO | TNT Thickness | ttb(90) (s) | 1/ttb (90) (s−1) |
|---|---|---|---|
| 100 nm | 180 nm | 50 | 0.020 |
| 600 nm | 1000 nm | 10 | 0.100 |
| 1000 nm | 2500 nm | 9 | 0.111 |
| Ti foil | - | ≥420 | ≤0.0024 |
| non-annealed TNT | - | ≥420 | ≤0.0024 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paušová, Š.; Kment, Š.; Zlámal, M.; Baudys, M.; Hubička, Z.; Krýsa, J. Transparent Nanotubular TiO2 Photoanodes Grown Directly on FTO Substrates. Molecules 2017, 22, 775. https://doi.org/10.3390/molecules22050775
Paušová Š, Kment Š, Zlámal M, Baudys M, Hubička Z, Krýsa J. Transparent Nanotubular TiO2 Photoanodes Grown Directly on FTO Substrates. Molecules. 2017; 22(5):775. https://doi.org/10.3390/molecules22050775
Chicago/Turabian StylePaušová, Šárka, Štěpán Kment, Martin Zlámal, Michal Baudys, Zdeněk Hubička, and Josef Krýsa. 2017. "Transparent Nanotubular TiO2 Photoanodes Grown Directly on FTO Substrates" Molecules 22, no. 5: 775. https://doi.org/10.3390/molecules22050775






