Synthesis of 1,2,3-Triazolo[4,5-h]quinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant Helicobacter pylori
Abstract
:1. Introduction
2. Results and Discussion
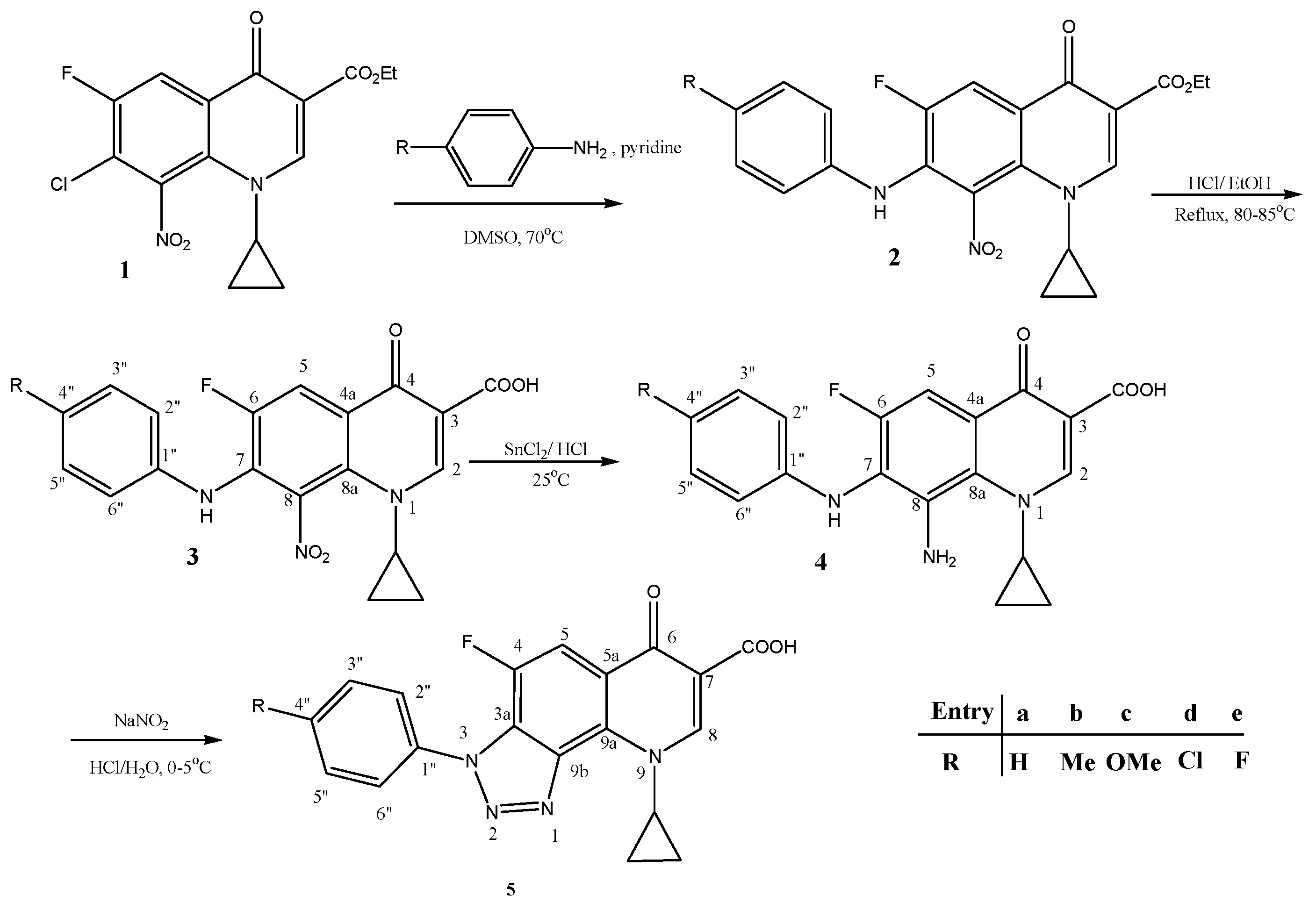
2.1. Synthesis of New Compounds
2.2. Antimicrobial Activity against Metronidazole-Resistant H. pylori
2.3. Inhibitory Effects of the Synthesized Compounds against H. pylori Urease Enzyme
2.4. Structural Activity Relationship Studies
3. Materials and Methods
3.1. Materials and Instruments
Synthesis of Novel Title Compounds 4b and 4c
Synthesis of Compound 1
Synthesis of New Compounds 4, 5 (b, c) (Scheme 1)
3.2. Microbiological Methods and Anti-Microbial Assays
3.2.1. Bacterial Strains and Growth Conditions
3.2.2. Antimicrobial Susceptibility Testing and Minimal Inhibitory Concentration Determination
3.2.3. Determination of In Vitro Interaction
3.3. Urease Inhibition Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar]
- Graham, D.Y. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: A model. Gastroenterology 1997, 113, 9183–9191. [Google Scholar] [CrossRef]
- Nomura, A.; Stemmermann, G.N.; Chyou, P.H.; Kato, I.; Perez-Perez, G.L.; Blaser, M.J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Ballal, M.; Lingadakai, R.; Mukhopadhyay, A. Determination of Helicobacter pylori virulence genes in clinical isolates of symptomatic patients from South Coastal Region of Karnataka—A preliminary work. Austin J. Gastroenterol. 2015, 2, 1031. [Google Scholar]
- Kalali, B.; Mejías-Luque, R.; Javaheri, A.; Gerhard, M. H. pylori virulence factors: Influence on immune system and pathology. Mediat. Inflamm. 2014, 2014, 6642–6651. [Google Scholar] [CrossRef] [PubMed]
- Konturek, J.W. Discovery by Jaworski of Helicobacter pylori and its pathogenetic role in peptic ulcer, gastritis and gastric cancer. Ann. Clin. Microbiol. Antimicrob. 2004, 10, 3–25. [Google Scholar]
- Tanih, N.F.; Dube, C.; Green, E.; Mkwetshana, N.; Clarke, A.M.; Ndip, L.M.; Ndip, R.N. An African perspective on Helicobacter pylori prevalence, drug resistance and alternative approaches to treatment. Ann. Trop. Med. Parasitol. 2009, 103, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, A.R.; Coelho, L.G.V. Helicobacter pylori and gastric malignancies. Helicobacter 2002, 7, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Megraud, F.; Lehours, P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 2007, 20, 280–322. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Wong, B.C. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 2007, 102, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Molina-Infante, J.; Gisbert, J.P.; O’Morain, C. Treatment of Helicobacter pylori infection. Helicobacter 2013, 18, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Giangaspero, A.; Losurdo, G.; Giorgio, F.; Amoruso, A.; de Francesco, V. Quadruple rescue therapy after first and second line failure for Helicobacter pylori treatment: Comparison between two tetracycline-based regimens. J. Gastrointest. Liver Dis. 2014, 23, 367–370. [Google Scholar]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.; Albrecht, P. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, R.M.; O’Morain, C.A.; O’Connor, H.J. Eradication of Helicobacter pylori: Recent advances in treatment. Fundam. Clin. Pharmacol. 2005, 19, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Gadhi, C.A.; Benharref, A.; Jana, M.; Lozniewski, A. Anti-Helicobacter pylori activity of Aristolochia paucinervis Pomel extracts. J. Ethnopharmacol. 2001, 75, 203–205. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Gelone, S.P. The newer fluoroquinolones. Infect. Dis. Clin. N. Am. 2004, 18, 691–716. [Google Scholar] [CrossRef] [PubMed]
- Roma, G.; Grossi, G.C.; Di Braccio, M.; Ghia, M.; Mattioli, F. 1,5-Benzodiazepines. IX. A new route to substituted 4H-[1,2,3]triazolo[4,3-a][1,5]benzodiazepin-5-amines with analgesic and/or anti-inflammatory activity. Eur. J. Med. Chem. 1991, 26, 489–496. [Google Scholar] [CrossRef]
- Chogtu, B.; Nayak, V.; Rajeshwari, S.D.; Bairy, K.L. Effect of fluoroquinolones on blood glucose levels in euglycemic Wistar rats. Pharmacologyonline 2010, 3, 918–922. [Google Scholar]
- Caliendo, G.; Di Carlo, R.; Greco, G.; Meli, R.; Novellino, E.; Perissutti, E.; Santagada, V. Synthesis and biological activity of benzotriazole derivatives structurally related to trazodone. Eur. J. Med. Chem. 1995, 30, 77–84. [Google Scholar] [CrossRef]
- Carta, A.; Piras, S.; Palomba, M.; Jabes, D.; Molicotti, P.; Zanetti, S. Anti-Mycobacterial activity of quinolones. Triazoloquinolones a new class of potent anti mycobacterial agents. Antiinfect. Agents Med. Chem. 2008, 7, 134–147. [Google Scholar] [CrossRef]
- Puakowska, A.; Bartulewicz, D.; Midura-Nowaczek, K. Aromatic benzotrazole amides synthesis and biological evaluation. Acta Pol. Pharm. 2005, 62, 59–64. [Google Scholar]
- Wan, J.; Tian, P.; Lv, N.; Zhu, H. Facile synthesis of novel benzotriazole derivatives and their antibacterial activities. J. Chem. Sci. 2010, 122, 597–606. [Google Scholar] [CrossRef]
- Zhanel, G.; Hoban, D.; Schurek, K.; Karlowsky, J. Role of efflux mechanisms on fluoroquinolone resistance in Streptococcus pneumoniae and Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2004, 24, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Vilaichone, R.K.; Gumnarai, P.; Ratanachu-ek, T.; Mahachai, V. Nationwide survey of Helicobacter pylori antibiotic resistance in Thailand. Diagn. Microbiol. Infect. Dis. 2013, 77, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Sheng, W.H.; Liou, J.M.; Wang, H.P.; Wu, M.S.; Lin, J.T.; Chang, S.C. Comparative in vitro antimicrobial susceptibility and synergistic activity of antimicrobial combinations against Helicobacter pylori isolates in Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiari, Y.M.; Qandil, A.M.; Al-Zoubi, R.M.; Alzweiri, M.H.; Darwish, R.M.; Shattat, G.F.; Al-Qirim, T.M. Synthesis and antibacterial activity of novel 7-haloanilino-8-nitrofluoroquinolone derivatives. Med. Chem. Res. 2012, 21, 1734–1740. [Google Scholar] [CrossRef]
- Al-Hiari, Y.; Al-Mazari, I.; Shakya, A.; Darwish, R.; Abu-Dahab, R. Synthesis and antibacterial properties of new 8-Nitro fluoroquinolones derivatives. Molecules 2007, 12, 1240–1258. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiari, Y.; Quandil, A.; Al-Zoubi, R.; Alzweiri, M.; Darwish, R.; Shattat, G.; Al-Quirim, T. 7-(3-Chlorophenylamino)-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid. Molbank 2010, 699, 1–3. [Google Scholar] [CrossRef]
- Abu-Qatouseh, L.; Abu-Sini, M.; Mayyas, A.; Al-Hiari, Y.; Darwish, R.; Aburjai, T. Synthesis of New Nitrofluoroquinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant H. pylori. Molecules 2017, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, A.; Al-Hadidi, K.; Aburjai, T.; Obeidat, F. Acute and subacute (20-d) oral dose toxicity study of modified fluoroquinolone compound 6C in BALB/c mice. Toxin Rev. 2015, 34, 129–135. [Google Scholar] [CrossRef]
- Arabiyat, S.; Al-Hiari, Y.; Bustanji, Y.; Zalloum, H.; Kasabri, V. In Vitro modulation of pancreatic lipase and proliferation of obesity related colorectal cancer cell line panel by novel synthetic triazoloquinolones. Rev. Roum. Chim. 2016, 61, 871–879. [Google Scholar]
- Al-Hiari, Y.; Arabiyat, S.; Bustanji, Y.; Kasabri, V.; Zalloum, H.; Mashalla, S.; Bashiti, R.; Maliti, J. Synthesis, docking Studies and evaluation of novel fluoroquinolones as potential pancreatic lipase Inhibitors and antiproliferative compounds. J. Enzym. Inhib. Med. Chem. 2017, in press. [Google Scholar]
- Al-Hiari, Y.; Shakya, A.; Alzweiri, M.; Aburjai, T.; Abu-Dahab, R. Synthesis and biological evaluation of substituted tetrahydro-1H-quino[7,8-b][1,4]benzodiazepine-3-carboxylic derivatives. FARMACIA 2014, 62, 570–588. [Google Scholar]
- Al-Hiari, Y.; Abu-Dahab, R.; El-Abadelah, M. Heterocycles [h]-fused onto 4-oxoquinoline-3-carboxylic acid, Part VIII [1]. Convenient synthesis and antimicrobial properties of substituted hexahydro[1,4]diazepino[2,3-h]quinoline-9-carboxylic acid and its tetrahydroquino[7,8-b]benzodiazepine analog. Molecules 2008, 13, 2880–2893. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.; Bazzoli, F.; El-Omar, E.M. Management of Helicobacter pylori infection—The Maastricht IV/Florence consensus report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Abu-Qatouseh, L.F.; Boutennone, H.; Boussouf, L.; Madani, K.; Shihab, P.; Al-Qaoud, K. In Vitro anti-Helicobacter pylori and urease inhibitory effects of polyphenolic extracts of local herbs from Algeria. IAJAA 2014, 3, 1–4. [Google Scholar]
- Mehmood, K.; Shoukat, S.; Hameed, Z.; Hussain, M.; Ahmed, S.; Shah, A.A.; Hameed, A.; Hasan, F. Evaluation of various strategies for isolation and culturing of Helicobacter pylori. Pak. J. Zool. 2011, 43, 427–435. [Google Scholar]
- Yakoob, J.; Abbas, Z.; Khan, R.; Naz, S.; Ahmad, Z.; Islam, M.; Awan, S.; Jafri, F.; Jafri, W. Prevalence of non Helicobacter pylori species in patients presenting with dyspepsia. BMC Gastroenterol. 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Klancnik, A.; Piskernik, S.; Jersek, B.; Mozina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Inoue, H.; Ishii, C.; Okazaki, Y.; Domon, H.; Utsui, Y. Effect of plaunotol in combination with clarithromycin or amoxicillin on Helicobacter pylori in vitro and in vivo. J. Antimicrob. Chemother. 2002, 50, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Satoh, H.; Iwahi, T.; Shimoyama, T.; Tamura, T. Potent inhibitory action of the gastric proton pump inhibitor lansoprazole against urease activity of Helicobacter pylori: Unique action selective for H. pylori cells. Antimicrob. Agents Chemother. 1993, 37, 769–774. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4c, 5 (a–d) are available from the authors. |
| Tested Compounds | Zones of Inhibition (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Isolates | Control Strain | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 4a | 25 | 45 | 35 | 40 | 46 | 43 | 40 | 16 | 11 | 38 | 41 | 42 | 40 |
| 4b | 25 | 46 | 38 | 44 | 48 | 45 | 42 | 21 | 15 | 42 | 40 | 44 | 49 |
| 4c | 24 | 38 | 34 | 37 | 35 | 38 | 35 | 20 | 14 | 29 | 37 | 35 | 45 |
| 4d | 15 | 35 | 30 | 35 | 38 | 36 | 44 | 18 | 20 | 25 | 35 | 40 | 44 |
| 4e | 15 | 35 | 34 | 40 | 45 | 42 | 43 | 17 | 12 | 30 | 38 | 36 | 43 |
| 5b | 10 | 15 | 12 | 10 | 20 | 12 | 13 | 15 | 7 | 17 | 18 | 16 | 21 |
| 5c | 16 | 30 | 25 | 28 | 29 | 30 | 22 | 15 | 7 | 25 | 29 | 28 | 35 |
| 5d | 22 | 22 | 20 | 19 | 24 | 20 | 16 | 14 | 8 | 20 | 22 | 20 | 32 |
| 5e | 20 | 25 | 15 | 15 | 24 | 17 | 14 | 13 | 7 | 19 | 24 | 22 | 25 |
| CIP | 20 | 60 | 0 | 50 | 53 | 65 | 59 | 0 | 0 | 64 | 50 | 55 | 65 |
| MTZ | 10 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Compounds | Minimum Inhibitory Concentration (μg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain Number | Control Strain | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 4a | 2.5 | 0.6 | 2.5 | 2.5 | 1.3 | 0.6 | 1.3 | 5 | 5 | 2.5 | 0.6 | 1.3 | 1.3 |
| 4b | 0.6 | 1.3 | 2.5 | 2.5 | 0.6 | 1.3 | 0.6 | 1.3 | 0.6 | 2.5 | 0.6 | 0.6 | 1.3 |
| 4c | 5 | 2.5 | 5 | 5 | 2.5 | 2.5 | 1.3 | 2.5 | 5 | 5 | 0.6 | 0.6 | 1.3 |
| 4d | 2.5 | 5 | 1.3 | 2.5 | 2.5 | 1.3 | 5 | 5 | 2.5 | 1.3 | 1.3 | 1.3 | 1.3 |
| 4e | 5 | 2.5 | 5 | 5 | 5 | 2.5 | 5 | 2.5 | 2.5 | 5 | 2.5 | 2.5 | 2.5 |
| 5b | 2.5 | 5 | 1.3 | 1.3 | 2.5 | 1.3 | 2.5 | 5 | 5 | 1.3 | 1.3 | 1.3 | 5 |
| 5c | 5 | 1.3 | 1.3 | 2.5 | 2.5 | 1.3 | 1.3 | 5 | 10 | 2.5 | 1.3 | 1.3 | 2.5 |
| 5d | 2.5 | 1.3 | 1.3 | 1.3 | 2.5 | 1.3 | 2.5 | 5 | 10 | 2.5 | 1.3 | 1.3 | 2.5 |
| 5e | 5 | 2.5 | 2.5 | 5 | 2.5 | 1.3 | 5 | 10 | 10 | 2.5 | 1.3 | 1.3 | 2.5 |
| CIP | 0.3 | 0.6 | 0.3 | 0.6 | 0.3 | 0.04 | 0.6 | 0.04 | 0.08 | 0.04 | 0.6 | 0.6 | 0.6 |
| MTZ | 64 | 64 | 64 | 32 | 128 | 64 | 128 | 32 | 64 | 64 | 128 | 64 | 128 |
| Compounds | FIC Values (Index) (MIC Combination) | FIC Mean | ||
|---|---|---|---|---|
| Strain Number | ||||
| 11 | 12 | Control Strain | ||
| 4a-MTZ | 2.039 (=) | 0.519 (+) | 0.519 (+) | 1.025 (=) |
| 4b-MTZ | 2.039 (=) | 1.019 (=) | 0.519 (+) | 1.192 (=) |
| 4c-MTZ | 4.078 (−) | 2.039 (=) | 0.519 (+) | 2.212 (=) |
| 4d-MTZ | 1.039 (=) | 0.519 (+) | 0.519 (+) | 0.692 (+) |
| 4e-MTZ | 1.078 (=) | 0.539 (+) | 0.539 (+) | 0.718 (+) |
| 5b-MTZ | 0.259 (*) | 0.519 (+) | 0.072 (*) | 0.283 (*) |
| 5c-MTZ | 0.519 (+) | 0.259 (*) | 0.269 (*) | 0.349 (*) |
| 5d-MTZ | 0.259 (*) | 0.519 (+) | 0.269 (*) | 0.349 (*) |
| 5e-MTZ | 0.519 (+) | 0.259 (*) | 0.134 (*) | 0.304 (*) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Sini, M.; Mayyas, A.; Al-Karablieh, N.; Darwish, R.; Al-Hiari, Y.; Aburjai, T.; Arabiyat, S.; Abu-Qatouseh, L. Synthesis of 1,2,3-Triazolo[4,5-h]quinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant Helicobacter pylori. Molecules 2017, 22, 841. https://doi.org/10.3390/molecules22050841
Abu-Sini M, Mayyas A, Al-Karablieh N, Darwish R, Al-Hiari Y, Aburjai T, Arabiyat S, Abu-Qatouseh L. Synthesis of 1,2,3-Triazolo[4,5-h]quinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant Helicobacter pylori. Molecules. 2017; 22(5):841. https://doi.org/10.3390/molecules22050841
Chicago/Turabian StyleAbu-Sini, Mohammad, Amal Mayyas, Nehaya Al-Karablieh, Rula Darwish, Yusuf Al-Hiari, Talal Aburjai, Shereen Arabiyat, and Luay Abu-Qatouseh. 2017. "Synthesis of 1,2,3-Triazolo[4,5-h]quinolone Derivatives with Novel Anti-Microbial Properties against Metronidazole Resistant Helicobacter pylori" Molecules 22, no. 5: 841. https://doi.org/10.3390/molecules22050841






