Enhanced Production of Gypenoside LXXV Using a Novel Ginsenoside-Transforming β-Glucosidase from Ginseng-Cultivating Soil Bacteria and Its Anti-Cancer Property
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of Strain Microbacterium sp. Gsoil 167
2.2. Fosmid Library Construction and Cloning of BglG167b
2.3. Phylogenetic Analysis of BglG167b Sequences
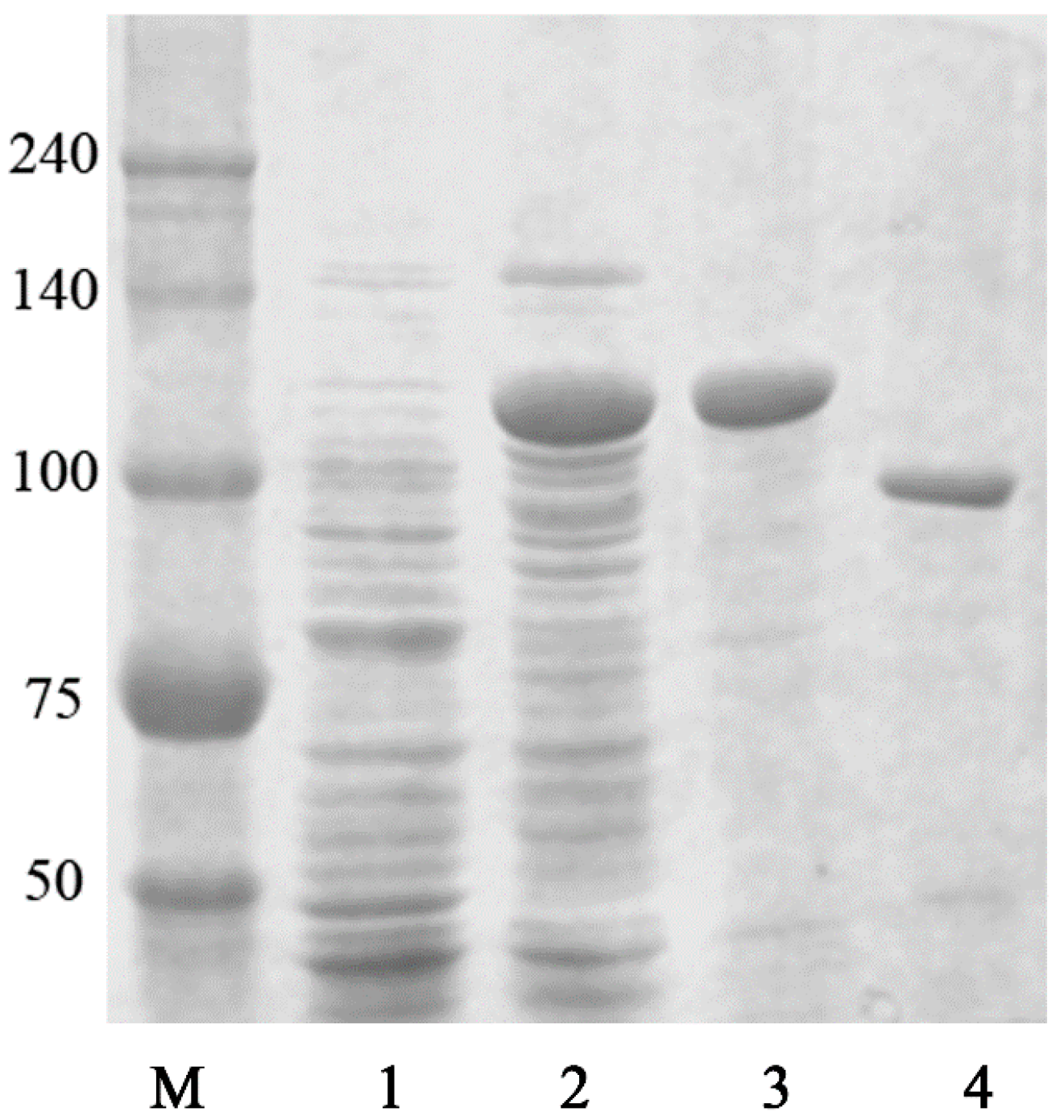
2.4. Purification of Recombinant BglG167b
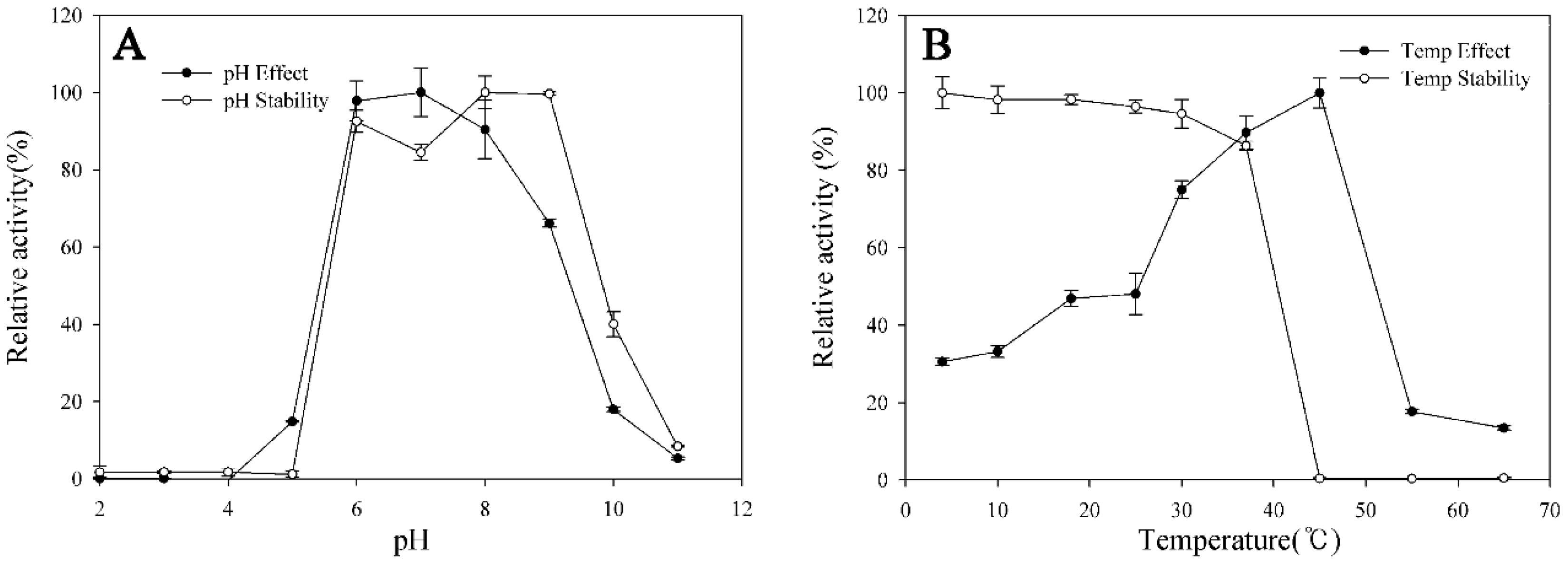
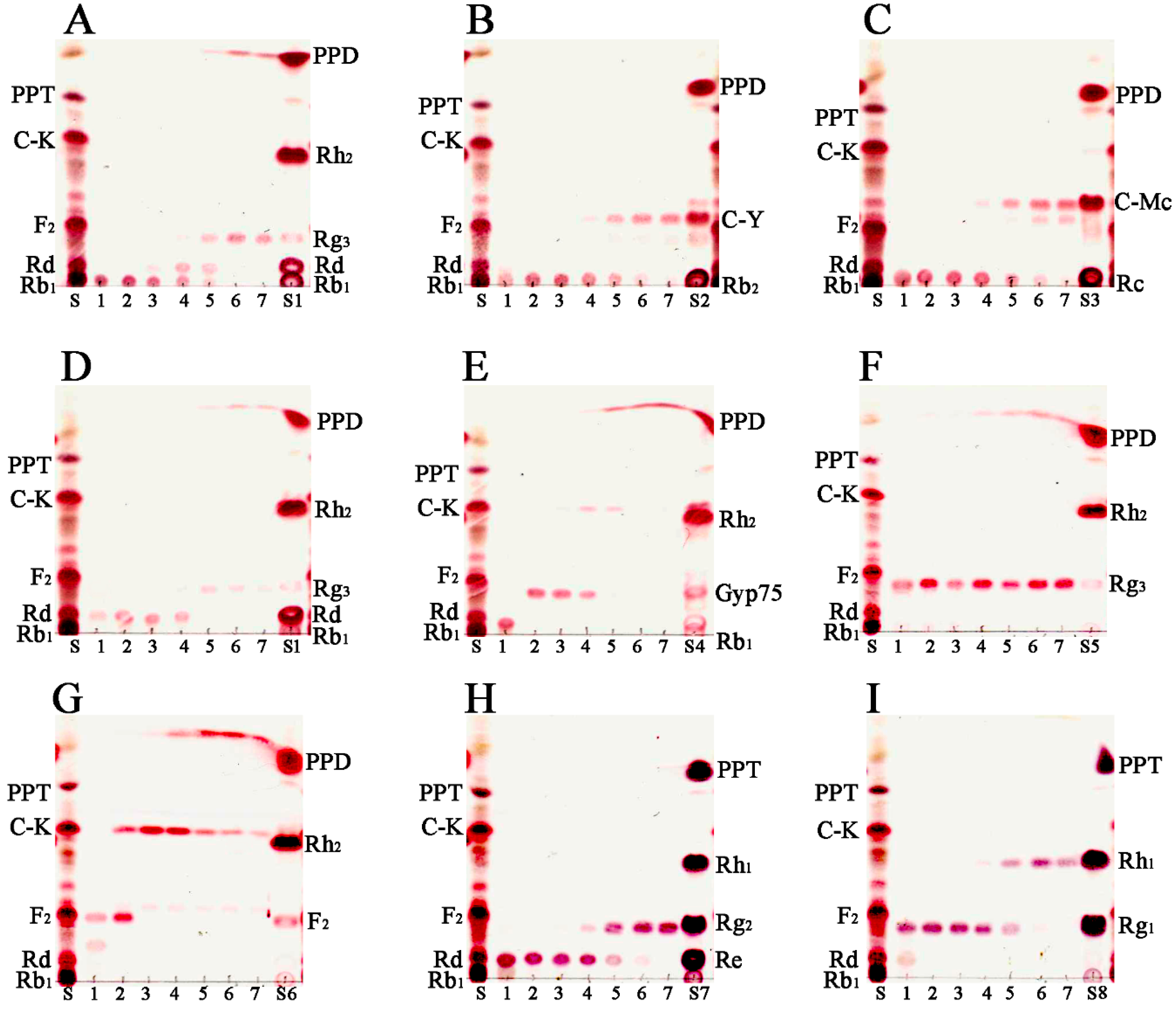
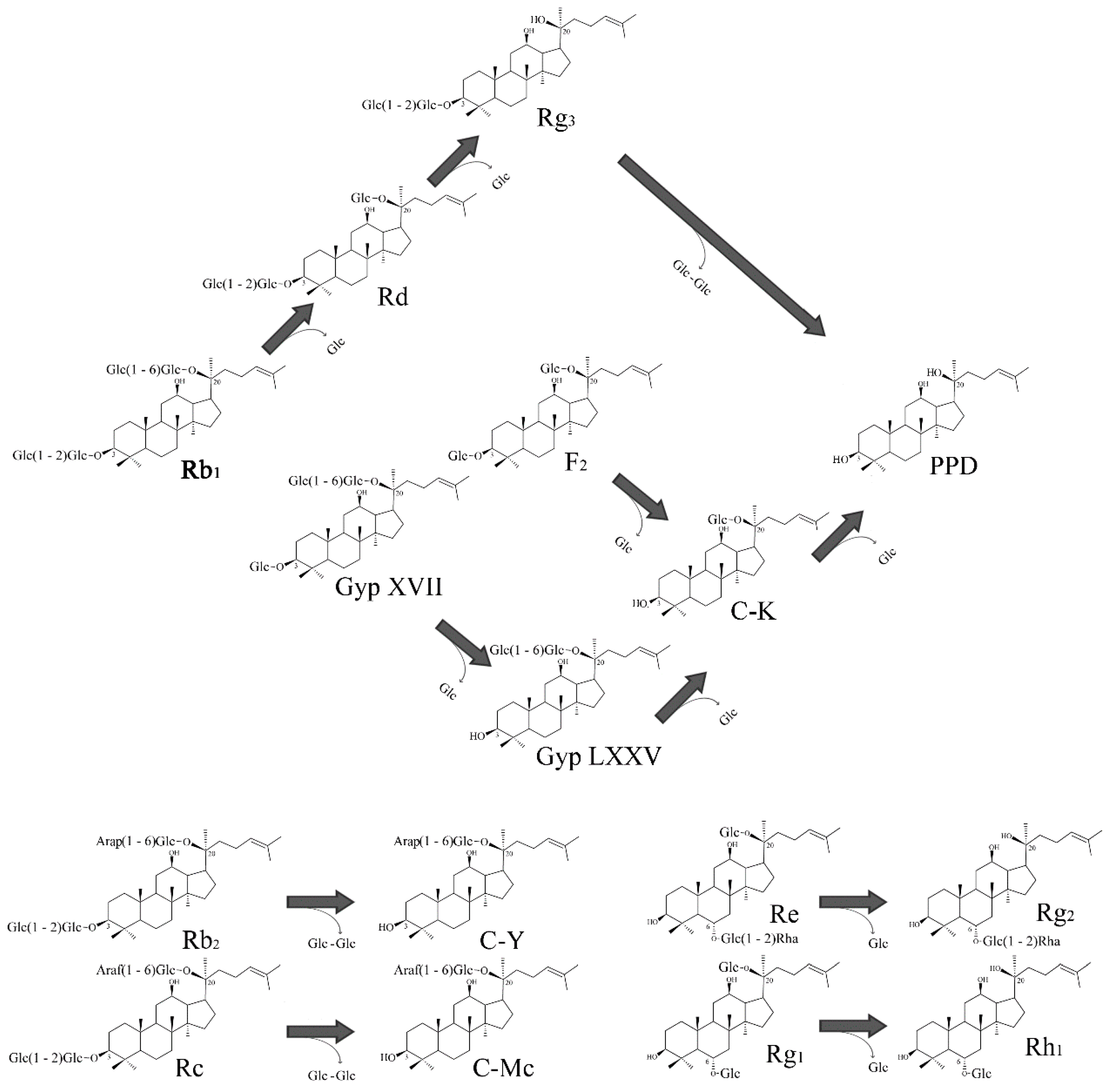
2.5. Transformation Characteristics of BglG167b
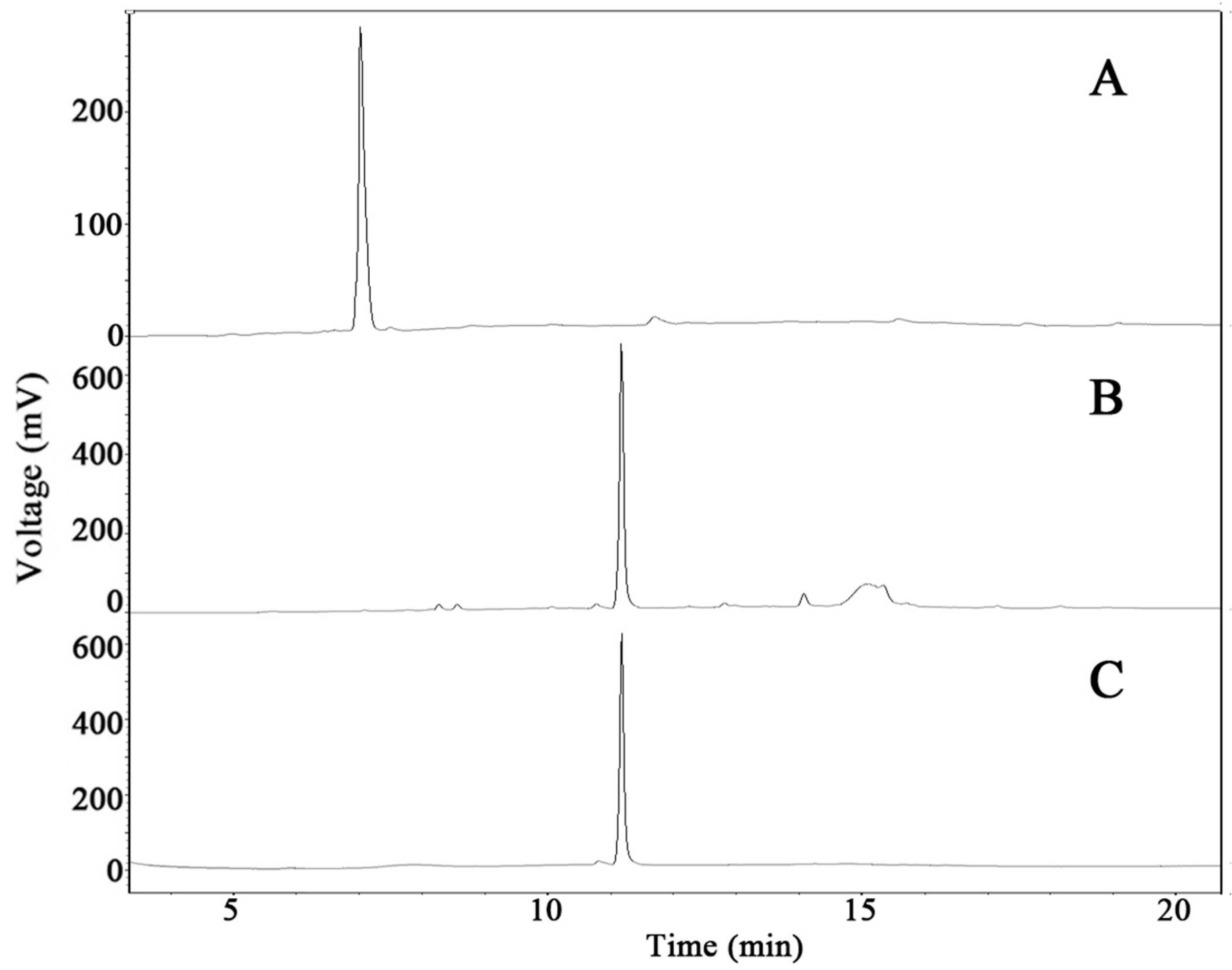
2.6. Scale-Up Production of GypLXXV and Purification
2.7. Cytotoxic Effect of GypLXXV on Cancer Cells
3. Materials and Methods
3.1. Materials
3.2. Fosmid Library Construction and Fosmid Sequencing
3.3. Phylogenetic Analysis of BglG167b
3.4. Molecular Cloning, Expression, and Purification of Recombinant BglG167b
3.5. Effect of pH, Temperature, Metal Ions, and Chemical Reagents on Enzyme Activity
3.6. Biotransformation Activity of Ginsenosides Using BglG167b
3.7. Gram-Scale GypLXXV Production Using Recombinant BglG167b
Preparation of Recombinant BglG167b Using High Cell Density Culture
3.8. Purification of GypLXXV
3.9. Cell Culture
3.10. MTT Assay
3.11. Analytic Methods
3.11.1. Thin Layer Chromatography (TLC) Analysis
3.11.2. High-Performance Liquid Chromatography (HPLC) Analysis
3.12. Nucleotide Sequence Accession Numbers
4. Conclusions
Supplementary Materials
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Shi, Y.; Sun, C.J.; Zheng, B.; Gao, B.; Sun, A.M. Simultaneous determination of ten ginsenosides in American ginseng functional foods and ginseng raw plant materials by liquid chromatography tandem mass spectrometry. Food. Anal. Methods 2013, 6, 112–122. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yuan, C.S. Potential role of ginseng in the treatment of colorectal cancer. Am. J. Chin. Med. 2008, 36, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Li, T.; Fong, C.M.V.; Chen, X.P.; Chen, X.J.; Wang, Y.T.; Huang, M.Q.; Lu, J.J. Saponins from Chinese Medicines as Anticancer Agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Liu, X.; Li, J.; Liu, S.; Qi, Y.; Xiong, Z.; Zhang, A.; Wiese, T.; Fu, X.; Gu, J.; et al. 20(S)-Protopanaxadiol-aglycone downregulation of the full-length and splice variants of androgen receptor. Int. J. Cancer 2013, 132, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, K.T.; Iseli, T.J.; Hoy, A.J.; George, J.; Grewal, T.; Roufogalis, B.D. Compound K modulates fatty acid-induced lipid droplet formation and expression of proteins involved in lipid metabolism in hepatocytes. Liver Int. 2013, 33, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.H.; Jin, Q.; Song, S.Z.; Wu, Y.L.; Bai, T.; Jiang, S.; Li, Q.; Yang, N.; Nan, J.X. Ginsenoside Rh2 downregulates LPS-induced NF-kappa B activation through inhibition of TAK1 phosphorylation in RAW 264.7 murine macrophage. Evid. Based Complement. Altern. Med. 2013, 2013, 646728. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. American ginseng: Potential structure-function relationship in cancer chemoprevention. Biochem. Pharmacol. 2010, 80, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Kim, B.S.; Kim, H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Zhang, Y.B.; Yang, X.W.; Yang, X.B.; Xu, W.; Xu, F.; Cai, S.Q.; Wang, Y.P.; Xu, Y.H.; Zhang, L.X. High-Performance Liquid Chromatography with Diode Array Detector and Electrospray Ionization Ion Trap Time-of-Flight Tandem Mass Spectrometry to Evaluate Ginseng Roots and Rhizomes from Different Regions. Molecules 2016, 21, 603. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jeon, J.N.; Jang, M.G.; Oh, J.Y.; Kwon, W.S.; Jung, S.K.; Yang, D.C. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J. Ginseng Res. 2014, 38, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Dong, L.; Wang, J.; Chen, S.L. Simultaneous determination of ten ginsenosides in panacis quinquefolii radix by ultra performance liquid chromatography and quality evaluation based on chemometric methods. Pharmazie 2011, 66, 553–559. [Google Scholar] [PubMed]
- Kang, M.S.; Baek, S.H.; Chun, Y.S.; Moore, A.Z.; Landman, N.; Berman, D.; Yang, H.O.; Morishima-Kawashima, M.; Osawa, S.; Funamoto, S.; et al. Modulation of lipid kinase PI4KIIalpha activity and lipid raft association of presenilin 1 underlies gamma-secretase inhibition by ginsenoside (20S)-Rg3. J. Biol. Chem. 2013, 288, 20868–20882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, G.J.; Wang, C.Z.; Wen, X.D.; Calway, T.; Li, Z.; He, T.C.; Du, W.; Bissonnette, M.; Musch, M.W.; et al. Compound K, a ginsenoside metabolite, inhibits colon cancer growth via multiple pathways including p53-p21 interactions. Int. J. Mol. Sci. 2013, 14, 2980–2995. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.F.; Eneroth, P.; Bruhn, J. Gynostemma pentaphyllum: Identification of major sapogenins and differentiation from Panax species. Eur. J. Pharm. Sci. 1999, 8, 187–191. [Google Scholar] [CrossRef]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar] [CrossRef] [PubMed]
- Open Reading Frame Finder. Available online: https://www.ncbi.nlm.nih.gov/orffinder/ (accessed on 17 May 2017).
- Compute pI/Mw tool, ExPASy Server. Available online: http://web.expasy.org/compute_pi/ (accessed on 17 May 2017).
- Castle, L.A.; Smith, K.D.; Morris, R.O. Cloning and sequencing of an Agrobacterium tumefaciens β-glucosidase gene involved in modifying a vir-inducing plant signal molecule. J. Bacteriol. 1992, 174, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Huang, Q.; Chang, L.; Sun, Q.W.; Zhou, J.G.; Lu, H. Cloning and characterization of two β-glucosidase/xylosidase enzymes from Yak rumen metagenome. Appl. Biochem. Biotechnol. 2012, 166, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Ali, A.; Chan, W.K.; Ho, V.; Lee, N.T. The cloning, expression and characterization of a cellobiase gene encoding a secretory enzyme from Cellulomonas biazotea. Gene 1998, 207, 79–86. [Google Scholar] [CrossRef]
- Feng, Y.; Duan, C.J.; Pang, H.; Mo, X.C.; Wu, C.F.; Yu, Y.; Hu, Y.L.; Wei, J.; Tang, J.L.; Feng, J.X. Cloning and identification of novel cellulase genes from uncultured microorganisms in rabbit cecum and characterization of the expressed cellulases. Appl. Microbiol. Biotechnol. 2007, 75, 319–328. [Google Scholar] [CrossRef] [PubMed]
- An, D.S.; Cui, C.H.; Lee, H.G.; Wang, L.; Kim, S.C.; Lee, S.T.; Jin, F.X.; Yu, H.S.; Chin, Y.W.; Lee, H.K.; et al. Identification and characterization of a novel Terrabacter ginsenosidimutans sp. nov. β-glucosidase that transforms ginsenoside Rb1 into the rare gypenosides XVII and LXXV. Appl. Environ. Microbiol. 2010, 76, 5827–5836. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Kim, S.C.; Im, W.T. Characterization of the ginsenoside-transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl. Microbiol. Biotechnol. 2013, 97, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.H.; Lee, J.H.; Hyun, Y.J.; Kim, D.H. Metabolism of ginsenoside Rb1 by human intestinal microflora and cloning of its metabolizing β-d-glucosidase from Bifidobacterium longum H-1. Biol. Pharm. Bull. 2012, 35, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Cui, C.H.; Yoon, M.H.; Kim, S.C.; Im, W.T. Bioconversion of major ginsenosides Rg1 to minor ginsenoside F1 using novel recombinant ginsenoside hydrolyzing glycosidase cloned from Sanguibacter keddieii and enzyme characterization. J. Biotechnol. 2012, 161, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.H.; Min, J.W.; Jin, Y.; Wang, C.; Kim, Y.J.; Yang, D.C. Enzymatic Biotransformation of Ginsenoside Rb1 to Compound K by Recombinant β-Glucosidase from Microbacterium esteraromaticum. J. Agric. Food Chem. 2012, 60, 3776–3781. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.H.; Min, J.W.; Sathiyamoorthy, S.; Yang, D.U.; Kim, Y.J.; Yang, D.C. Biotransformation of ginsenosides Re and Rg1 into ginsenosides Rg2 and Rh1 by recombinant β-glucosidase. Biotechnol. Lett. 2012, 34, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.H.; Min, J.W.; Yang, D.U.; Kim, Y.J.; Yang, D.C. Enzymatic biotransformation of ginsenoside Rb1 to 20(S)-Rg3 by recombinant β-glucosidase from Microbacterium esteraromaticum. Appl. Microbiol. Biotechnol. 2012, 94, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.C.; Zhang, H.; Zhang, L.X.; Liu, Z.; Sun, G.Z.; Lei, J.; Qin, Y.X.; Zheng, Y.N.; Li, X.; Pan, H.Y. Biotransformation of ginsenoside Rf to Rh1 by recombinant β-glucosidase. Molecules 2009, 14, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Liu, Q.M.; Kim, J.K.; Sung, B.H.; Kim, S.G.; Kim, S.C.; Im, W.T. Identification and Characterization of Mucilaginibacter sp. QM49 β-Glucosidase and its use in Producing the Pharmaceutically Active Minor Ginsenosides, Rh1(S) and Rg2(S). Appl. Environ. Microbiol. 2013, 79, 5788–5798. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Cui, C.H.; Liu, Q.; Yoon, M.H.; Kim, S.C.; Im, W.T. Mass production of the ginsenoside Rg3(S) through the combinative use of two glycoside hydrolases. Food Chem. 2013, 141, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.G.; Xue, J.J.; Sun, M.Q.; Wang, C.Y.; Liu, L.; Zhang, D.L.; Lee, M.R.; Gu, L.J.; Wang, C.L.; Wang, Y.B.; et al. Highly selective microbial transformation of major ginsenoside Rb1 to gypenoside LXXV by Esteya vermicola CNU120806. J. Appl. Microbiol. 2012, 113, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Kim, J.K.; Kim, S.C.; Im, W.T. Characterization of a ginsenoside-transforming β-glucosidase from Paenibacillus mucilaginosus and its application for enhanced production of minor ginsenoside F2. PLoS ONE 2014, 9, e85727. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cui, C.H.; Park, S.C.; Kim, J.K.; Yu, H.S.; Jin, F.X.; Sun, C.; Kim, S.C.; Im, W.T. Identification and characterization of a ginsenoside-transforming β-glucosidase from Pseudonocardia sp. Gsoil 1536 and its application for enhanced production of minor ginsenoside Rg2(S). PLoS ONE 2014, 9, e96914. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.Q.; Na, J.R.; Bang, M.H.; Kim, M.K.; Yang, D.C. Conversion of major ginsenoside Rb1 to 20(S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry 2008, 69, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Lee, S.K.; Kim, N.D.; Park, M.K.; Kim, C.K.; Park, J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000, 63, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, X.M.; Wang, Q.J.; Zhang, D.L.; Fang, Z.M.; Wang, C.Y.; Wang, Z.; Sun, B.S.; Wu, H.; Sung, C.K. Enzymatic preparation of 20(S,R)-protopanaxadiol by transformation of 20(S,R)-Rg3 from black ginseng. Phytochemistry 2010, 71, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Cui, C.H.; Kim, J.K.; Jin, F.X.; Kim, S.C.; Im, W.T. Enzymatic Biotransformation of Ginsenoside Rb1 and Gypenoside XVII into Ginsenosides Rd and F2 by Recombinant β-glucosidase from Flavobacterium johnsoniae. J. Ginseng Res. 2012, 36, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.L.; Dong, W.W.; Wu, S.Q.; Jiang, J.; Yang, D.C.; Li, D.H.; Quan, L.H. Biotransformation of gypenoside XVII to compound K by a recombinant β-glucosidase. Biotechnol. Lett. 2016, 38, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.S.T.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.G.; Huang, Y.; Cui, D.D.; Huang, X.B.; Mao, S.H.; Ji, L.L.; Song, H.B.; Yi, C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer 2009, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Moon, J.; Song, Y.; Viet, P.Q.; Phuc, P.V.; Lee, J.M.; Yi, T.H.; Cho, M.; Cho, S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012, 321, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Im, W.T.; Kim, S.C. Composition for Preventing or Treating Cervical Cancer including Gypenoside LXXV. Patent WO2017026641 A1, 16 February 2017. [Google Scholar]
- Carbohydrate-Active enZymes Database. Available online: http://www.cazy.org (accessed on 17 May 2017).
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kimura, M. The Neutral Theory of Molecular Evolution; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Gypenoside XVII and Gypenoside LXXV are available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.-H.; Kim, D.J.; Jung, S.-C.; Kim, S.-C.; Im, W.-T. Enhanced Production of Gypenoside LXXV Using a Novel Ginsenoside-Transforming β-Glucosidase from Ginseng-Cultivating Soil Bacteria and Its Anti-Cancer Property. Molecules 2017, 22, 844. https://doi.org/10.3390/molecules22050844
Cui C-H, Kim DJ, Jung S-C, Kim S-C, Im W-T. Enhanced Production of Gypenoside LXXV Using a Novel Ginsenoside-Transforming β-Glucosidase from Ginseng-Cultivating Soil Bacteria and Its Anti-Cancer Property. Molecules. 2017; 22(5):844. https://doi.org/10.3390/molecules22050844
Chicago/Turabian StyleCui, Chang-Hao, Da Jung Kim, Suk-Chae Jung, Sun-Chang Kim, and Wan-Taek Im. 2017. "Enhanced Production of Gypenoside LXXV Using a Novel Ginsenoside-Transforming β-Glucosidase from Ginseng-Cultivating Soil Bacteria and Its Anti-Cancer Property" Molecules 22, no. 5: 844. https://doi.org/10.3390/molecules22050844



