Recent Progress in Nanomaterial-Based Electrochemical Biosensors for Cancer Biomarkers: A Review
Abstract
:1. Introduction
2. Metal Nanoparticle-Based Biosensors for Cancer Biomarkers
2.1. AuNP-Modified Electrodes as Biosensors
2.2. Metal Nanoparticles as Signaling Labels
3. Carbon Nanotube-Based Biosensors for Cancer Biomarkers
3.1. CNT-Modified Electrodes as Biosensors
3.2. CNTs as Signaling Labels
4. Graphene-Based Biosensors for Cancer Biomarkers
4.1. Graphene-Modified Electrodes
4.2. Graphene As Signaling Labels
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Le, M.H.; Jimenez, C.; Chainet, E.; Stambouli, V. A label-free impedimetric DNA sensor based on a nanoporous SnO2 film: Fabrication and detection performance. Sensors 2015, 15, 10686–10704. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene-gold nanoparticles hybrid—Synthesis, functionalization, and application in a electrochemical and surface-enhanced Raman scattering biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Yu, Y.; Li, T.; Li, G.; Anzai, J. Enzymatically regulated peptide pairing and catalysis for the bioanalysis of extracellular prometastatic activities of functionally linked enzymes. Sci. Rep. 2016, 6, 25362. [Google Scholar] [CrossRef] [PubMed]
- Ibupoto, Z.H.; Khun, K.; Willander, M. A selective iodide ion sensor electrode based on functionalized ZnO nanotubes. Sensors 2013, 13, 1984–1997. [Google Scholar] [CrossRef] [PubMed]
- Bougrini, M.; Baraket, A.; Jamshaid, T.; Aissari, A.E.; Bausells, J.; Zabala, M.; Bari, N.E.; Bouchikhi, B.; Jaffrezic-Renault, N.; Abdelhamid, E.; et al. Development of a novel capacitance electrochemical biosensor based on silicon nitride for ochratoxin A detection. Sens. Actuators B 2016, 234, 446–452. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; Shi, H.; Song, Z.; Zhao, Z.; Anzai, J.; Osa, T.; Chen, Q. Multi-walled carbon nanotubes-based glucose biosensor prepared by a layer-by-layer technique. Mater. Sci. Eng. C 2006, 26, 113–117. [Google Scholar] [CrossRef]
- Zheng, D.; Vashist, S.K.; Dykas, M.M.; Saha, S.; Al-Rubeaan, K.; Lam, E.; Luong, J.H.T.; Sheu, F. Graphene versus multi-walled carbon nanotubes for electrochemical glucose biosensing. Materials 2013, 6, 1011–1027. [Google Scholar] [CrossRef]
- Pagaduan, J.V.; Sahore, V.; Woolley, A.T. Applications of microfluidics and microchip electrophoresis for potential clinical biomarker analysis. Anal. Bioanal. Chem. 2015, 407, 6911–6922. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Esimbekova, E.N.; Kratasyuk, V.A. Rapid biosensing tools for cancer biomarkers. Biosens. Bioelectron. 2017, 87, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tang, B.C.; Yang, W.J.; Liu, Y.; Zhao, Y.L.; Li, M. Integration of a bio-chip technique with technetium-99m labeling provides zeptomolar sensitivity in liver cancer biomarker detection. Anal. Methods 2015, 7, 1622–1626. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Liu, K.; Wei, Y.; Wang, X.; Duan, Y. Novel signal-enhancing immunoassay for ultrasensitive biomarker detection based on laser-induced fluorescence. Anal. Chem. 2015, 87, 2959–2965. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Baldo, M.A.; Ortega, F.G.; Pereira, S.V.; Bertolino, F.A.; Serrano, M.J.; Lorente, J.A.; Raba, J.; Messina, G.A. Nanostructured platform integrated into a microfluidic immunosensor coupled to laser-induced fluorescence for the epithelial cancer biomarker determination. Microchem. J. 2016, 128, 18–25. [Google Scholar] [CrossRef]
- Snyder, C.M.; Alley, W.R., Jr.; Campos, M.I.; Svoboda, M.; Goetz, J.A.; Vasseur, J.A.; Jacobson, S.C.; Novotny, M.V. Complementary glycomic analyses of sera derived from colorectal cancer patients by MALDI-TOF-MS and microchip electrophoresis. Anal. Chem. 2016, 88, 9597–9605. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Cheng, F.; Lu, X.; Duan, Y.; Wang, X. Untargeted saliva metabonomics study of breast cancer based on ultra performance liquid chromatography coupled to mass spectrometry with HILIC and RPLC separations. Talanta 2016, 158, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Duan, L.; Liu, M.; Dong, X. An epitope imprinted polymer with affinity for kinenogen fragments prepared by metal coordination interaction for cancer biomarker analysis. J. Mater. Chem. B 2016, 4, 7464–7471. [Google Scholar] [CrossRef]
- Filella, X.; Foj, L. Prostate cancer detection and prognosis: From prostate specific antigen (PSA) to exosomal biomarkers. Int. J. Mol. Sci. 2016, 17, 1784. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Yin, X. The diagnostic value of serum CEA, NSE and MMP-9 for on-small cell lung cancer. Open Med. 2016, 11, 59–62. [Google Scholar]
- Siangproh, W.; Dungchai, W.; Rattanarat, P.; Chailapakul, O. Nanoparticle-based electrochemical detection in conventional and miniaturized systems and their bioanalytical applications: A review. Anal. Chim. Acta 2011, 690, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Hu, S. Electrochemical sensors based on graphene materials. Microchim. Acta 2011, 175, 1–9. [Google Scholar]
- Fang, Y.; Zhang, D.; Qin, X.; Miao, Z.; Takahashi, S.; Anzai, J.; Chen, Q. A non-enzymatic hydrogen peroxide sensors based on poly(vinyl alcohol)—Multiwalled carbon nanotubes—Platinum nanoparticles hybrids modified glassy carbon electrode. Electrochim. Acta 2012, 70, 266–271. [Google Scholar] [CrossRef]
- Takahashi, S.; Abiko, N.; Anzai, J. Redox response of reduced graphene oxide-modified glassy carbon electrodes to hydrogen peroxide and hydrazine. Materials 2013, 6, 1840–1850. [Google Scholar] [CrossRef]
- Zhang, D.; Fang, Y.; Miao, Z.; Ma, M.; Du, X.; Takahashi, S.; Anzai, J.; Chen, Q. Direct electrodeposition of reduced graphene oxide and dendritic copper nanoclusters on glassy carbon electrode for electrochemical detection of nitride. Electrochim. Acta 2013, 107, 656–663. [Google Scholar] [CrossRef]
- Perféxou, M.; Turner, A.; Merkoçi, A. Cancer detection using nanoparticle-based sensors. Chem. Soc. Rev. 2012, 41, 2606–2622. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.V.; Doble, M.; Verma, R.S. Nanomaterials for early detection of cancer biomarkers with special emphasis on gold nanoparticles in immunoassay/sensors. Biosens. Bioelectron. 2015, 68, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, A.; Voccia, D.; Palchetti, I.; Marrazza, G. Electrochemical, electrochemiluminescence, and photoelectrochemical aptamer-based nanostructured sensors for biomarker analysis. Biosensors 2016, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Sankara, V.S.P.K.; Jayanthi, A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Yehezkeli, O.; Tel-Vered, R.; Raichlin, S.; Willner, I. Nano-engineered Flavin-dependent glucose dehydrogenase/gold nanoparticle-modified electrodes for glucose sensing and biofuel cell applications. ACS Nano 2011, 5, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, M.; Elviri, L.; Careri, M.; Mangia, A.; Mori, G. A voltammetric immunosensor based on nanobiocomposite materials for the determination of alpha-fetoprotein in serum. Biosens. Bioelectron. 2011, 26, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Gan, N.; Cao, Y.; Hu, F.; Li, T.; Zheng, L. An ultrasensitive electrochemical immunosensor for alpha-fetoprotein using an envision complex-antibody copolymer as a sensitive label. Materials 2012, 5, 2757–2772. [Google Scholar] [CrossRef]
- Elshafey, R.; Tavares, A.C.; Siaj, M.; Zourob, M. Electrochemical impedance immunosensor based on gold nanoparticles-protein G for the detection of cancer marker epidermal growth factor receptor in human plasma and brain tissue. Biosens. Bioelectreon. 2013, 50, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qi, L.; Gao, Z.; Zu, X.; Chen, M. A novel electrochemical immunosensor for prostate-specific antigen based on noncovalent nanocomposite of ferrocene monocarboxylic acid with graphene oxide. Anal. Lett. 2014, 47, 2266–2280. [Google Scholar] [CrossRef]
- Wang, W.; Fan, X.; Xu, S.; Davis, J.J.; Luo, X. Low fouling label-free DNA sensor based on polyethylene glycols decorated with gold nanoparticles for the detection of breast cancer biomarkers. Biosens. Bioelectron. 2015, 71, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, Z. Highly stable electrochemical immunosensor for carcinoembryonic antigen. Biosens. Bioelectron. 2012, 35, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Anzai, L. Phenylboronic acid monolayer-modified electrodes sensitive to sugars. Langmuir 2005, 21, 5102–5107. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Anzai, J. Redox reactions of ferricyanide ions in layer-by-layer deposited polysaccharide films: A significant effect of the type of polycation in the films. Langmuir 2007, 23, 7378–7384. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, H.; Chen, Z.; Mu, X.; Guo, L. Aptamer biosensor for label-free square-wave voltammetry detection of angiogenin. Biosens. Bioelectron. 2011, 30, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Karaboğa, M.N.S.; Şimşek, C.S.; Sezgintürk, M.K. AuNPs modified, disposable, ITO based biosensor: Early diagnosis of heat shock protein 70. Biosens. Bioelectron. 2016, 84, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.C.; Krause, C.E.; Sotzing, G.A.; Rusling, J.F. Inkjet-printed gold nanoparticle electrochemical arrays on plastic. Application to immunodetection of a cancer biomarker protein. Phys. Chem. Chem. Phys. 2011, 13, 4888–4894. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, Y.; Huang, J.; Zhao, Z.; Xu, X.; Anzai, J.; Osa, T.; Chen, Q. Amperometric choline biosensors prepared by layer-by-layer deposition of choline oxidase on the Prussian blue-modified platinum electrode. Talanta 2006, 70, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Sato, K.; Anzai, J. Layer-by-layer assembled thin films composed of carboxyl-terminated poly(amidoamine) dendrimer as a pH-sensitive nano-device. J. Colloid Interface Sci. 2008, 326, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Barsan, M.M.; Brett, C.M.A. Recent advances in layer-by-layer strategies for biosensors incorporating metal nanoparticles. Trend Anal. Chem. 2016, 79, 286–296. [Google Scholar] [CrossRef]
- Chikkaveeraish, B.V.; Mani, V.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Microfluidic electrochemical immunoarray for ultrasensitive detection of two cancer biomarker proteins in serum. Biosens. Bioelectron. 2011, 26, 4477–4483. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.E.; Otieno, B.A.; Bishop, G.W.; Phadke, G.; Choquette, L.; Lalla, R.V.; Peterson, D.E.; Rusling, J.F. Ultrasensitive microfluidic array for serum pro-inflammatory cytokines and C-reactive protein to assess oral mucositis risk in cancer patients. Anal. Bioanal. Chem. 2015, 407, 7239–7243. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, X.; Chen, L.; Zhu, Y.; Zhang, X. Ultrasensitive IL-6 electrochemical immunosensors based on Au nanoparticles-graphene-silica biointerface. Colloid Surf. B 2014, 116, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Hu, Y.; Liu, A.; Chen, W.; Lin, X.; Yu, X. Label-free electrochemical immunosensor based on multi-functional gold nanoparticles-polydopamine-thionine-graphene oxide nanocomposites film for determination of alpha-fetoprotein. J. Electroanal. Chem. 2014, 712, 89–95. [Google Scholar] [CrossRef]
- Ortega, F.G.; Fernández-Baldo, M.A.; Serrano, M.J.; Messina, G.A.; Lorente, J.A.; Raba, J. Epithelial cancer biomarker EpCAM determination in peripheral blood samples using a microfluidic immunosensor based in silver nanoparticles as platform. Sens. Actuators B 2015, 221, 248–256. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Rashidi, M.R.; Barar, J.; Aghaie, M.; Mohammadnejad, D.; Ramazani, A.; Karami, P.; Coukos, G.; Omidi, Y. An ultra-sensitive impedimetric immunosensor for detection of the serum oncomarker CA-125 in ovarian cancer patients. Nanoscale 2015, 7, 3768–3779. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Yang, H.; Zhang, Y.; Yu, J.; Ge, S.; Yan, M.; Song, X. Branched zinc oxide nanorods arrays modified paper electrode for electrochemical immunosensing by combining biocatalytic precipitation reaction and competitive immunoassay mode. Biosens. Bioelectron. 2015, 74, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wan, Y.; Deng, S.; Yang, S.; Su, Y.; Fan, C.; Aldalbahi, A.; Zuo, X. Aptamer-initiated on-particle template-independent enzymatic polymerization (aptamer-OTEP) for electrochemical analysis of tumor biomarkers. Biosens. Bioelectron. 2016, 86, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.K.; Vaze, A.; Shen, M.; Rusling, J.F. High-throughput electrochemical microfluidic immunoarray for multiplexed detection of cancer biomarker proteins. ACS Sens. 2016, 1, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chu, C.; Shen, L.; Deng, W.; Yan, M.; Ge, S.; Yu, J.; Song, X. An ultrasensitive electrochemical immunosensor based on the catalytical activity of MoS2-Au composite using Ag nanospheres as labels. Sens. Actuators B 2015, 206, 30–36. [Google Scholar] [CrossRef]
- Sun, G.; Liu, H.; Zhang, Y.; Yu, J.; Yan, M.; Song, X.; He, W. Gold nanorods-paper electrode based enzyme-free electrochemical immunoassay for prostate specific antigen using porous zinc oxide spheres-silver nanoparticles nanocomposites as labels. New J. Chem. 2015, 39, 6062–6067. [Google Scholar] [CrossRef]
- Huang, J.; Tian, J.; Zhao, Y.; Zhao, S. Ag/Au nanoparticles coated graphene electrochemical sensor for ultrasensitive analysis of carcinoembryonic antigen in clinical immunoassay. Sens. Actuators B 2015, 206, 570–576. [Google Scholar] [CrossRef]
- Parnsubsakul, A.; Safitri, R.E.; Rijiravanich, P.; Surareungchai, W. Electrochemical assay of proteolytically active prostate specific antigen based on anodic stripping voltammetry of silver enhanced gold nanoparticle labels. J. Electroanal. Chem. 2017, 785, 125–130. [Google Scholar] [CrossRef]
- Ilkhani, H.; Sarparast, M.; Noori, A.; Bathaie, S.Z.; Mousavi, M.F. Electrochemical aptamer/antibody based sandwich immunosensor for the detection of EGFR, a cancer biomarker, using gold nanoparticles as a signaling probe. Biosens. Bioelectron. 2015, 74, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, Q.; He, J.; Li, T.; Zhao, Y.; Cai, Y.; Du, B.; Qian, Z.; Yang, M. Electrochemical immunosensors for cancer biomarker with signal amplification based on ferrocene functionalized iron oxide nanoparticles. Biosens. Bioelectron. 2011, 26, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Anzai, J. Ferrocene-containing polyelectrolyte multilayer film-covered electrodes: Electrocatalytic determination of ascorbic acid and use inner blocking layers to improve the upper detection limit of the electrodes. Anal. Bioanal. Chem. 2004, 380, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhou, W.; Guo, J.; Wang, R.; Liang, R. Amperometric sensor based ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal. Biochem. 2009, 385, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Anzai, J. Recent progress in ferrocene-modified thin films and nanoparticles for biosensors. Materials 2013, 6, 5742–5762. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, T.; Li, H.; Ye, Z.; Chen, Z.; Li, G. A novel electrochemical immunosensor for golgi protein 73 assay. Electrochem. Commun. 2014, 42, 6–8. [Google Scholar] [CrossRef]
- Wang, B.; Anzai, J. Recent progress in lectin-based biosensors. Materials 2015, 8, 8590–8607. [Google Scholar] [CrossRef]
- Wang, B.; Anzai, J. Recent progress in electrochemical HbA1c sensors: A Review. Materials 2015, 8, 1187–1203. [Google Scholar] [CrossRef]
- Sharma, P.S.; Wojnarowicz, A.; Sosnowska, M.; Benincori, T.; Noworyta, K.; D’Souza, F.; Kutner, W. Potentiometric chemosensor for neopterin, a cancer biomarker, using an electrochemically synthesized molecularly imprinted polymer as the recognition unit. Biosens. Bioelectron. 2016, 77, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, S.; Wang, B. Boronic acid-based lectin mimics (boronolectins) that can recognize cancer biomarker, the Thomsen-Friedenrich antigen. ChemBioChem 2010, 11, 2245–2246. [Google Scholar] [CrossRef] [PubMed]
- Anzai, J. Recent progress in electrochemical biosensors based on phenylboronic acid and derivatives. Mater. Sci. Eng. C 2016, 67, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Yang, X.; Xi, X. Cathode photoelectrochemical immunoassay based on analyte-induced formation of exciton trapping for carcinoembryonic antigen detection. J. Electroanal. Chem. 2015, 757, 192–197. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Jin, Z.; Wang, J.; Li, Y.; Jiang, K.; Fan, S. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Biomolecule-functionalized carbon nanotubes: Applications in nanobioelectronics. ChemPhysChem 2004, 5, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Song, Z.; Li, J.; Yang, Y.; Shi, H.; Wu, B.; Anzai, J.; Osa, T.; Chen, Q. A highly-sensitive L-lactate biosensor based on sol-gel film combined with multi-walled carbon nanotubes (MWCNTs) modified electrode. Mater. Sci. Eng. C 2007, 27, 29–34. [Google Scholar] [CrossRef]
- Yan, Y.M.; Baravik, I.; Yehezkeli, O.; Willner, I. Integrated electrically contacted glucose oxidase/carbon nanotube electrodes for the bioelectrocatalyzed detection of glucose. J. Phys. Chem. C 2008, 112, 17883–17888. [Google Scholar] [CrossRef]
- Braik, M.; Barsan, M.M.; Dridi, C.; Ali, M.B.; Brett, C.M.A. Highly sensitive amperometric enzyme biosensor for detection of superoxide based on conducting polymer/CNT modified electrodes and superoxide dismutase. Sens. Actuators B 2016, 236, 574–582. [Google Scholar] [CrossRef]
- Teixeira, S.; Conlan, R.S.; Guy, O.J.; Sales, M.G.F. Novel single-wall carbon nanotubes screen-printed electrode as an immunosensor for human chorionic gonadotropin. Electrochim. Acta 2014, 136, 323–329. [Google Scholar] [CrossRef]
- Nawaz, M.A.H.; Rauf, S.; Catanante, G.; Nawas, M.H.; Nunes, G.; Marty, J.L.; Hayat, A. One step assembly of thin films of carbon nanotubes on screen printed interface for electrochemical aptasensing of breast cancer biomarker. Sensors 2016, 16, 1651. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Willander, M.; Sharma, J.G.; Malhotra, B.D. A solution processed carbon nanotube modified conducting paper sensor for cancer detection. J. Mater. Chem. B 2015, 3, 9305–9314. [Google Scholar] [CrossRef]
- Niu, Y.; He, J.; Li, Y.; Zhao, Y.; Xia, C.; Yuan, G.; Zhang, L.; Zhang, Y.; Yu, C. Determination of 2,3-sialylated glycans in human serum using a glassy carbon electrode modified with carboxylated multiwalled carbon nanotubes, a polyamidoamine dendrimer, and a glycan-recognizing lectin from Maackia Amurensis. Microchim. Acta 2016, 183, 2337–2344. [Google Scholar] [CrossRef]
- Paul, K.B.; Singh, V.; Vanjari, S.R.K.; Singh, S.G. One step biofunctionalized electrospun multiwalled carbon nanotubes embedded zinc oxide nanowire interface for highly sensitive detection of carcinoma antigen-125. Biosens. Bioelectron. 2017, 88, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Munge, B.S.; Fisher, J.; Millord, L.N.; Krause, C.E.; Dowd, R.S.; Rusling, J.F. Sensitive electrochemical immunosensor for matrix metalloproteinase-3 based on single-wall carbon nanotubes. Analyst 2010, 135, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Sardesai, N.P.; Barron, J.C.; Rusling, J.F. Carbon nanotube microwell array for sensitive electrochemiluminescent detection of cancer biomarker proteins. Anal. Chem. 2011, 83, 6698–6703. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Kichambare, P.D.; Star, A. Carbon nanotube field-effect-transistor-based biosensors. Adv. Mater. 2007, 19, 1439–1451. [Google Scholar] [CrossRef]
- Heller, I.; Janssens, A.M.; Männik, J.; Minot, E.D.; Lemay, S.G.; Dekker, C. Identifying the mechanism of biosensing with carbon nanotube transistor. Nano Lett. 2008, 8, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Lerner, M.B.; D’Souza, J.; Pazina, T.; Dailey, J.; Goldsmith, B.R.; Ronbinson, M.K.; Johnson, A.T.C. Hybrids of a genetically engineered antibody and a carbon nanotube transistor for detection of prostate cancer biomarkers. ACS Nano 2012, 6, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Freitas, A.C.; Amaral, J.P.; Rocha-Santos, T.A.P.; Cardoso, S.; Duarte, A.C. Disposable immunosensors for C-reactive protein based on carbon nanotubes field effect transistors. Talanta 2013, 108, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, W.; Shen, J.; Jiang, Y.; Han, E.; Dong, X.; Huang, J. Carbohydrate derivative-functionalized biosensing toward highly sensitive electrochemical detection of cell surface glycan expression as cancer biomarker. Biosens. Bioelectron. 2015, 74, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Jeong, B.; Choi, J.; Rahman, M.A. Ultrasensitive nanoimmunosensor by coupling non-covalent functionalized graphene oxide platform and numerous ferritin labels on carbon nanotubes. Biosens. Bioelectron. 2016, 80, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Rahman, M.A.; Rhee, C.K. Amplified electrochemical detection of a cancer biomarker by enhanced precipitation using horseradish peroxidase attached on carbon nanotubes. Anal. Chem. 2012, 84, 6407–6415. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Han, J.; Zhou, Y.; Yang, Z.; Chai, Y.; Yuan, R. Highly sensitive impedimetric immunosensor based on single-walled carbon nanotubes as labels and bienzyme biocatalyzed precipitation as enhancer for cancer biomarker detection. Biosens. Bioelectron. 2014, 55, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lin, D.; Wu, J.; Ding, L.; Ju, H.; Yan, F. Nanogold-enriched carbon nanohorn label for sensitive electrochemical detection of biomarker on a disposable immunosensor. Electroanalysis 2013, 25, 1044–1049. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Aizen, R.; Yehezkeli, O.; Willner, I. Graphene oxide/nucleic-acid-stabilized silver nanoclusters: Functional hybrid materials for optical aptamer sensing and multiplexed analysis of pathogenic DNAs. J. Am. Chem. Soc. 2013, 135, 11832–11839. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Duan, H. 2D and 3D graphene materials: Preparation and bioelectrochemical applications. Biosens. Bioelectron. 2015, 65, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Dai, Z.; Lin, Y.; Du, D. Electrochemical immunoassays based on graphene: A review. Electroanal 2016, 28, 4–12. [Google Scholar] [CrossRef]
- Wang, S.; Cazelles, R.; Liao, W.C.; Vázquez-González, M.; Zoabi, A.; Abu-Reziq, R.; Willner, I. Mimicking horseradish peroxidase and NADH peroxidase by heterogeneous Cu2+-modified graphene oxide nanoparticles. Nano Lett. 2017, 17, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Yoshikawa, H.; Tamiya, E.; Yasin, H.M.; Ahmed, M.U. A highly sensitive gold nanoparticle bioprobe based electrochemical immunosensor using screen printed graphene biochip. RSC Adv. 2014, 4, 58460–58466. [Google Scholar] [CrossRef]
- Idegami, K.; Chikae, M.; Kerman, K.; Nagatani, N.; Yuhi, T.; Endo, T.; Tamiya, E. Gold nanoparticle-based redox enhancement for sensitive detection of human chorionic gonadotropin hormone. Electroanal 2008, 20, 14–21. [Google Scholar] [CrossRef]
- Viet, N.X.; Chikae, M.; Ukita, Y.; Maehashi, K.; Matsumoto, K.; Tamiya, E.; Viet, P.H.; Takamura, Y. Gold-linked electrochemical immunoassay on single-walled carbon nanotube for highly sensitive detection of human chorionic gonadotropin hormone. Biosens. Bioelectron. 2013, 42, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Teixeria, S.; Conlan, R.S.; Guy, O.J.; Sales, M.G.F. Label-free human chorionic gonadotropin detection at pictogram levels using oriented an antibodies bound to graphene screen-printed electrodes. J. Mater. Chem. B 2014, 2, 1852–1855. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. Paper-based microfluidic immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal. Chem. 2013, 85, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, P.; Hui, K.M.; Kang, Y. Paper-based microfluidic immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2014, 52, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Srivastava, S.; Yadav, B.Y.; Lee, S.H.; Sharma, J.G.; Doval, D.C.; Malhorta, B.D. Reduced graphene oxide modified smart conducting paper for cancer sensor. Biosens. Bioelectron. 2015, 73, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Li, S.; Turner, A.P.F. Electrochemical immunosensor with N-doped graphene-modified electrode for label-free detection of the breast cancer biomarker CA 15-3. Biosens. Bioelectron. 2013, 43, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Teixeria, S.; Burwell, B.; Castaing, A.; Gonzalez, D.; Conlan, R.S.; Guy, O.J. Epitaxial graphene immunosensor for human chorionic gonadotropin. Sens. Acatuators B 2014, 190, 723–729. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Wang, T.; Li, D.; Xi, F.; Wang, J.; Wang, E. Three-dimensional electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on monolithic macroporous graphene foam. Biosens. Bioelectron. 2015, 65, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, Z.; Liu, N.; Ma, Z. A label-free immunosensor based on graphene nanocomposites for simultaneous multiplexed electrochemical determination of tumor markers. Biosens. Bioelectron. 2014, 53, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yuan, R.; Chai, Y.; Zhou, Y.; Jiang, W.; Su, H.; Che, X.; Li, J. Horseradish peroxidase-functionalized Pt hollow nanospheres and multiple redox probes as tracer labels for a sensitive simultaneous multianalyte electrochemical immunoassay. Chem. Commun. 2010, 46, 6750–6752. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, M.; Li, H. Lebel-free electrochemical detection of cancer marker based on graphene-cobalt hexacyanoferrate nanocomposite. J. Electroanal. Chem. 2011, 655, 50–55. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wei, Q.; Wu, H.; Dou, J.; Li, H. Ionic liquid functionalized graphene based immunosensor for sensitive detection of carbohydrate antigen 15–3 integrated with Cd2+-functionalized nanoporous TiO2 as labels. Biosens. Bioelectron. 2014, 59, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Samanman, S.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Highly-sensitive label-free electrochemical carcinoembryonic antigen immujnosensor based on a novel Au nanoparticles-graphene-chitosan nanocomposite cryogel electrode. Anal. Chim. Acta 2015, 853, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, X.; Han, J.; Ma, J.; Ma, Z. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens. Bioelectron. 2013, 50, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, N.; Ma, Z. Prussian blue-gold nanoparticles-ionic liquid functionalized reduced graphene oxide nanocomposite as label for ultrasensitive electrochemical immunoassay of alpha-fetoprotein. Anal. Chim. Acta 2014, 829, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Yu, J.; Ge, S.; Song, X. All-graphene composite materials for signal amplification toward ultrasensitive electrochemical immunosensing of tumor marker. Biosens. Bioelectron. 2015, 71, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Si, Y.; Zhang, N.; Sun, Z.; Wu, H.; Lin, Y. Platinum nanocatalysts loaded on graphene oxide-dispersed carbon nanotubes with greatly enhanced peroxidase-like catalysis and electrocatalysis activities. Nanoscale 2014, 6, 8107–8116. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Si, Y.; Sun, Z.; Li, S.; Lin, Y. Recyclable enzyme mimic of cubic Fe3O4 nanoparticles loaded on graphene oxide-dispersed carbon nanotubes with enhanced peroxidase-like catalysis and electrocatalysis. J. Mater. Chem. B 2014, 2, 4442–4448. [Google Scholar] [CrossRef]
- Du, D.; Wang, L.; Shao, Y.; Wang, J.; Engelhard, M.H.; Lin, Y. Functionalized graphene oxide as a nanocarrier in a multienzyme labeling amplification strategy for ultrasensitive electrochemical immunoassay of phosphorylated p53 (S392). Anal. Chem. 2011, 83, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Bai, J.; Shi, X.; Zeng, Y.; Xian, Y.; Hou, J.; Jin, L. Graphene oxide as nanocarrier for sensitive electrochemical immunoassay of clenbuterol based on labeling amplification strategy. Talanta 2013, 107, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, K.; Wang, J.; Shao, K.; Fu, T.; Shao, F.; Lu, D.; Liang, J.; Foda, M.F.; Han, H. Solid-state voltammetry-based electrochemical immunosensor for Escherichia coli using graphene oxide-Ag nanoparticle composites as labels. Analyst 2013, 138, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.G.; Muthoosamy, K.; Shipton, F.N.; Pandikumar, A.; Rameshkumar, P.; Huang, N.M.; Manickam, S. The biogenic synthesis of a reduced graphene oxide-silver (RGO-Ag) nanocomposite and its dual applications as an antibacterial agent and cancer biomarker sensor. RSC Adv. 2016, 6, 36576–36587. [Google Scholar]

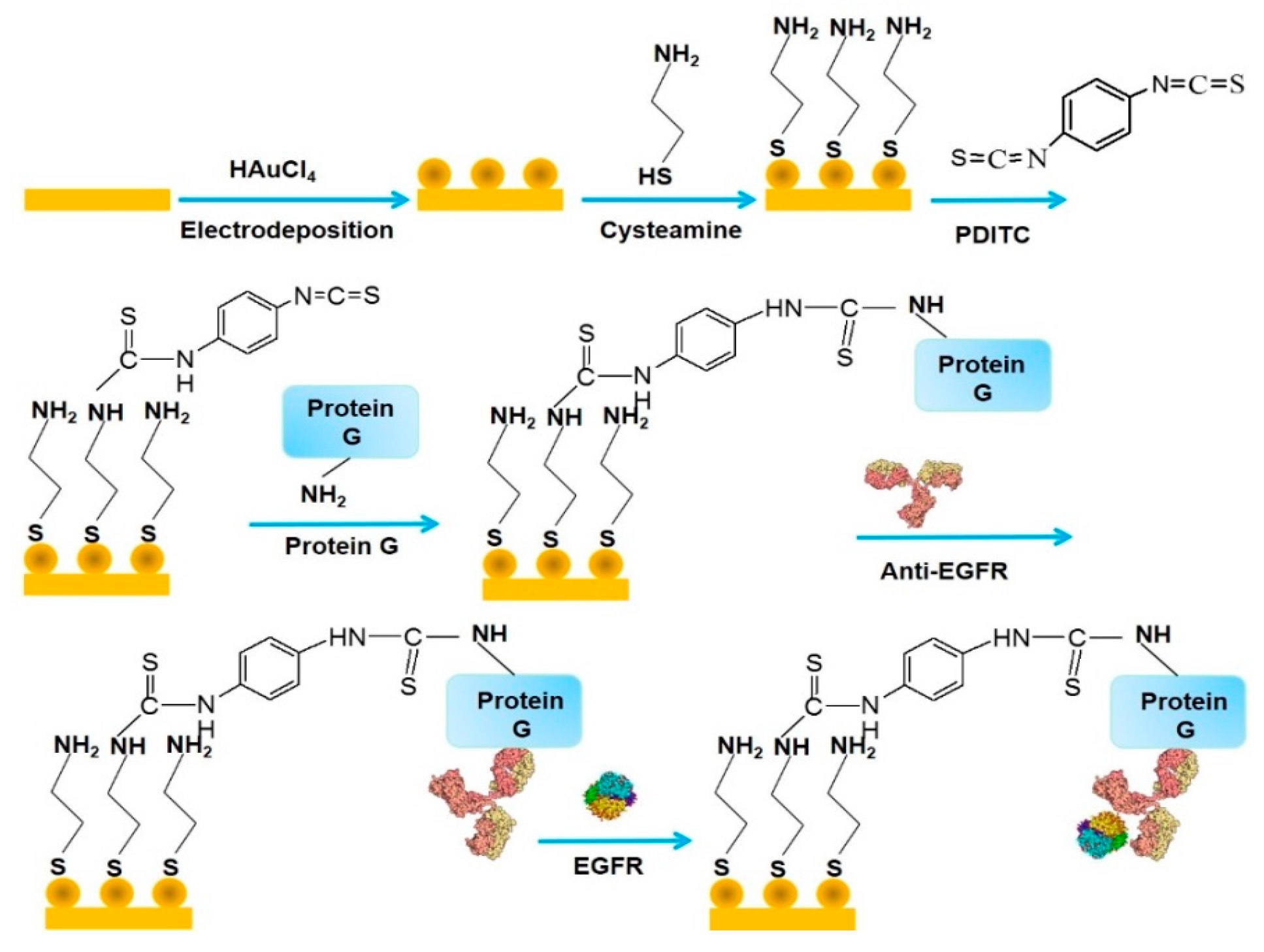
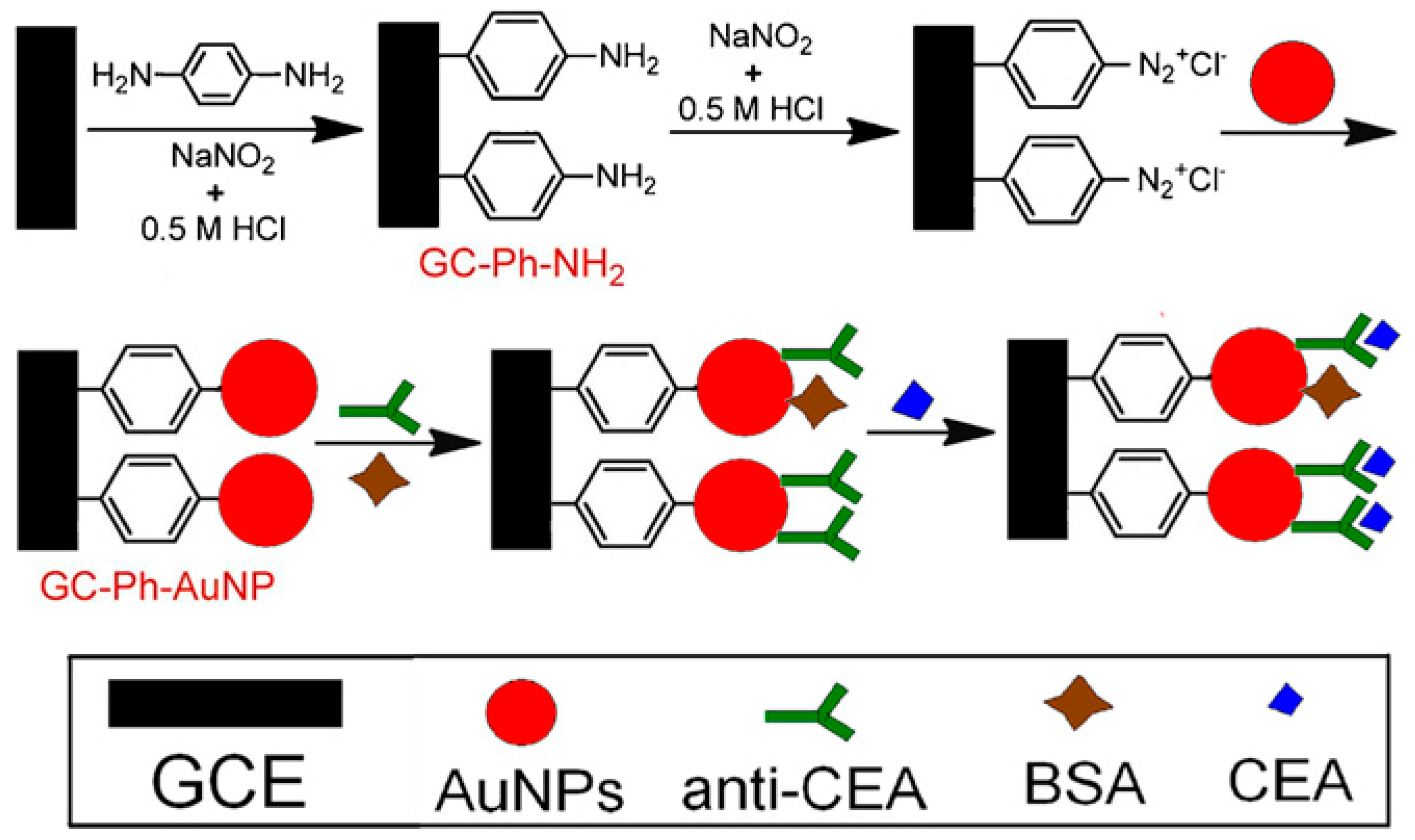

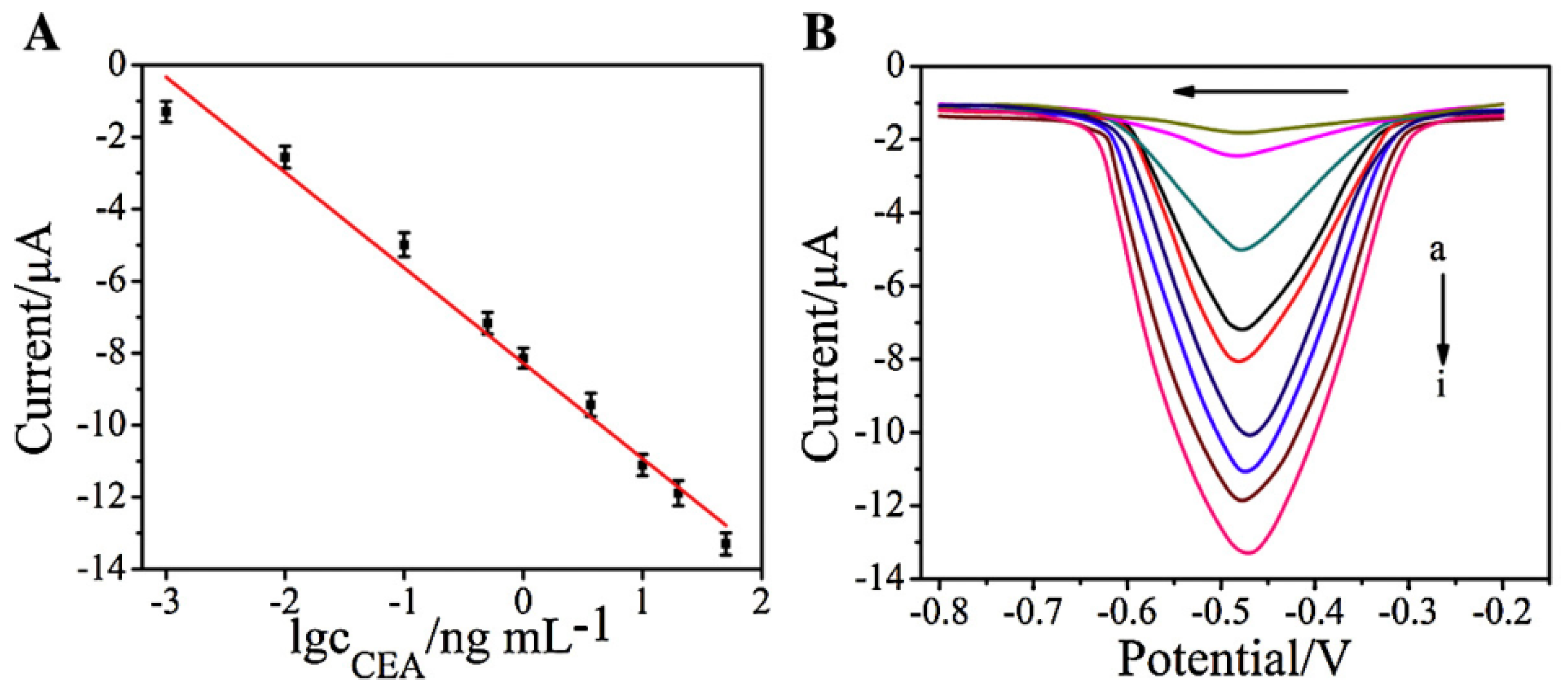

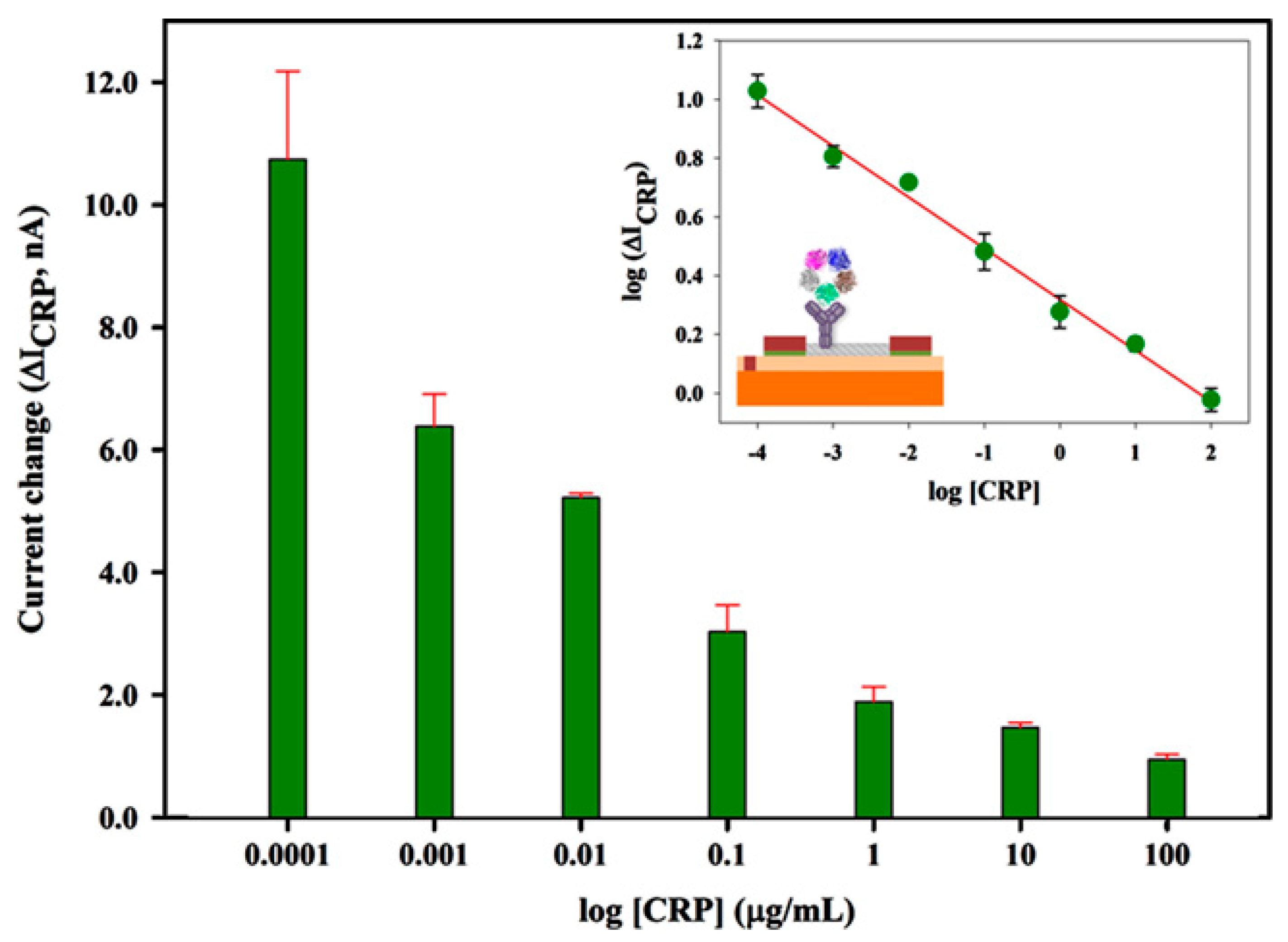
| Nanomaterials Used | Electrode | Transduction Method | Analyte | Detection Range | LOD | Ref. |
|---|---|---|---|---|---|---|
| protein A/AuNPs | GCE | voltammetry | AFP | 5–80 ng mL−1 | 3.7 ng mL−1 | [31] |
| protein G/AuNPs | AuE | voltammetry | AFP | 0.005–0.2 ng mL−1 | 2 pg mL−1 | [32] |
| protein G/AuNPs | AuE | impedimetry | EGFR | 0.001–1000 ng mL−1 | 0.34 pg mL−1 | [33] |
| ferrocene/AuNPs | GCE | voltammetry | PSA | 0.002–10 ng mL−1 | 0.5 pg mL−1 | [34] |
| DNA/AuNPs | GCE | impedimetry | BRCA1 | 50 fM–1 nM | 1.72 fM | [35] |
| AuNPs | GCE | voltammetry | CEA | 10 fg mL−1–100 ng mL−1 | 3 fg mL−1 | [36] |
| AuNPs | ITO | impedimetry | HSP70 | 1–166 fg mL−1 | 0.0618 fg mL−1 | [40] |
| glutathione/AuNPs | SPCE | amperometry | IL-6 | 0.3–20 pg mL−1 | 0.3 pg mL−1 | [45] |
| glutathione/AuNPs | SPCE | amperometry | PSA | 0.225–5 pg·mL−1 | 0.1 pg mL−1 | [45] |
| graphene/AuNPs | ITO | amperometry | IL-6 | 1–40 pg mL−1 | 0.3 pg mL−1 | [47] |
| Th/PDA/GO/AuNPs | GCE | voltammetry | AFP | 0.1–150 ng mL−1 | 0.03 ng mL−1 | [48] |
| CdSe/silica/AuNPs | AuE | impedimetry | CA-125 | 0–0.1 U mL−1 | 0.0016 U mL−1 | [50] |
| ZnO/AuNPs | GO paper | voltammetry | AFP | 0.0002–500 ng mL−1 | 0.08 pg mL−1 | [51] |
| GOx/AgNPs | GCE | voltammetry | CEA | 0.001–50 ng mL−1 | 0.27 pg mL−1 | [54] |
| ZnO/AgNPs | AuNRs paper | voltammetry | PSA | 0.004–60 ng mL−1 | 1.5 pg mL−1 | [55] |
| Ag/AuNPs | GCE | voltammetry | CEA | 0.01–120 ng mL−1 | 8 pg mL−1 | [56] |
| MCF/AuNPs | GCE | voltammetry | CEA | 0.05–1000 pg mL−1 | 0.024 pg mL−1 | [57] |
| antibody/AuNPs | GCE | voltammetry | EGFR | 1–40 ng mL−1 | 50 pg mL−1 | [58] |
| ferrocene/Fe3O4 | GCE | voltammetry | PSA | 0.01–40 ng mL−1 | 2 pg mL−1 | [59] |
| CdSe QDs | GCE | voltammetry | GP73 | 20–5000 pM | 12 pM | [63] |
| Nanomaterials Used | Electrode | Transduction Method | Analyte | Detection Range | LOD | Ref. |
|---|---|---|---|---|---|---|
| SWCNTs | SPE | impedimetry | hCG | 0.01–100 ng mL−1 | - | [75] |
| aptamer/MWCNTs | SPE | impedimetry | mucine | 0.1–2 U mL−1 | 0.02 U mL−1 | [76] |
| PEDOT/CNTs | filter paper | amperometry | CEA | 2–15 ng mL−1 | 1 ng mL−1 | [77] |
| MWCNTs | GCE | voltammetry | si-Gly | 10 fg·mL−1–50 ng mL−1 | 3 fg mL−1 | [78] |
| MWCNTs/ZnO | GCE | voltammetry | CA125 | 0.001–1000 U mL−1 | 0.00113 U mL−1 | [79] |
| aligned SWCNTs | PGE | amperometry | MMP-3 | 4–300 pg mL−1 | 4 pg mL−1 | [80] |
| antibody/CNTs | FET | I-VG | OPN | 0.001–1000 ng mL−1 | 1 pg mL−1 | [84] |
| SWCNTs | FET | ID-VD | CRP | 0.0001–100 μg mL−1 | 0.1 ng mL−1 | [85] |
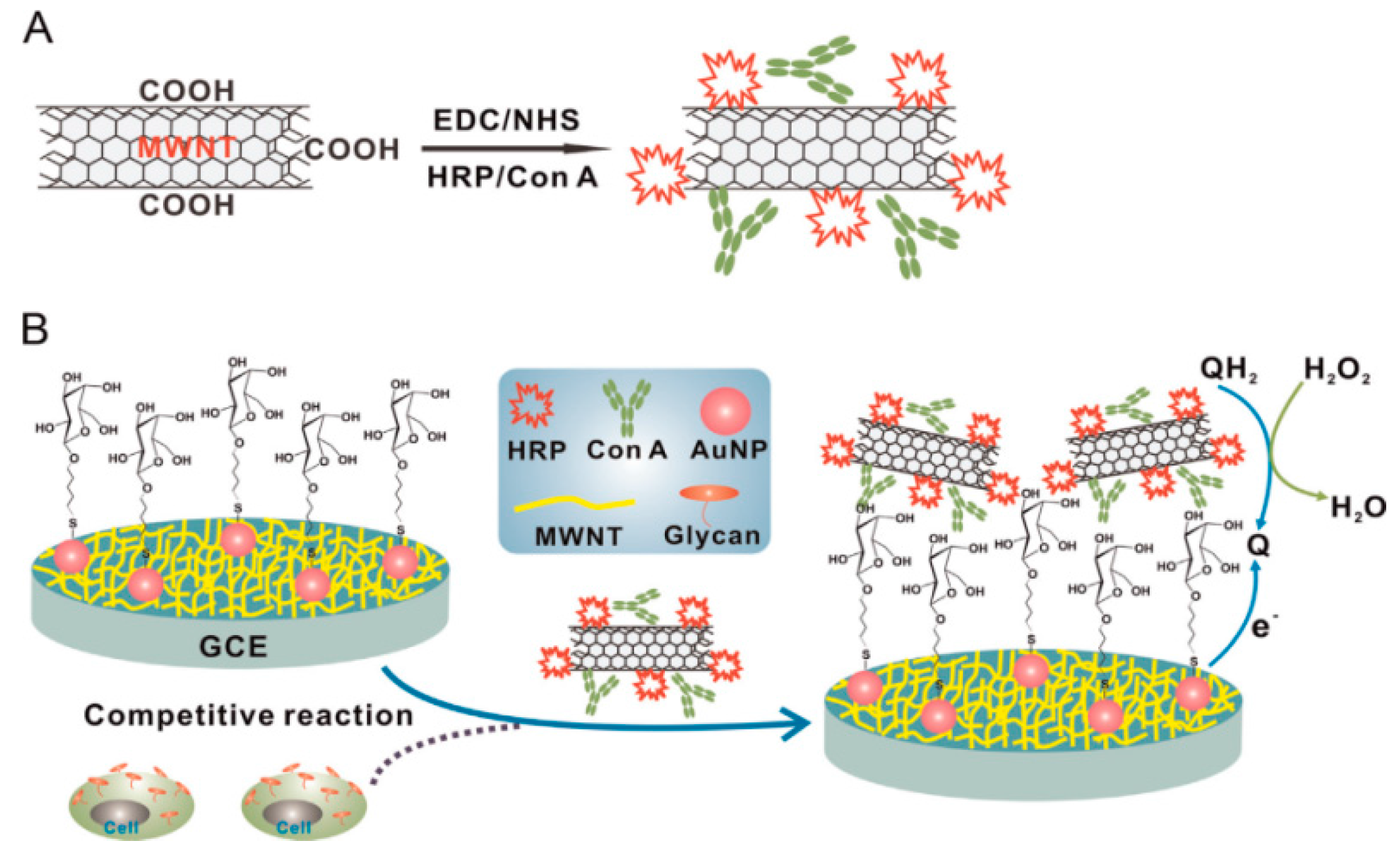
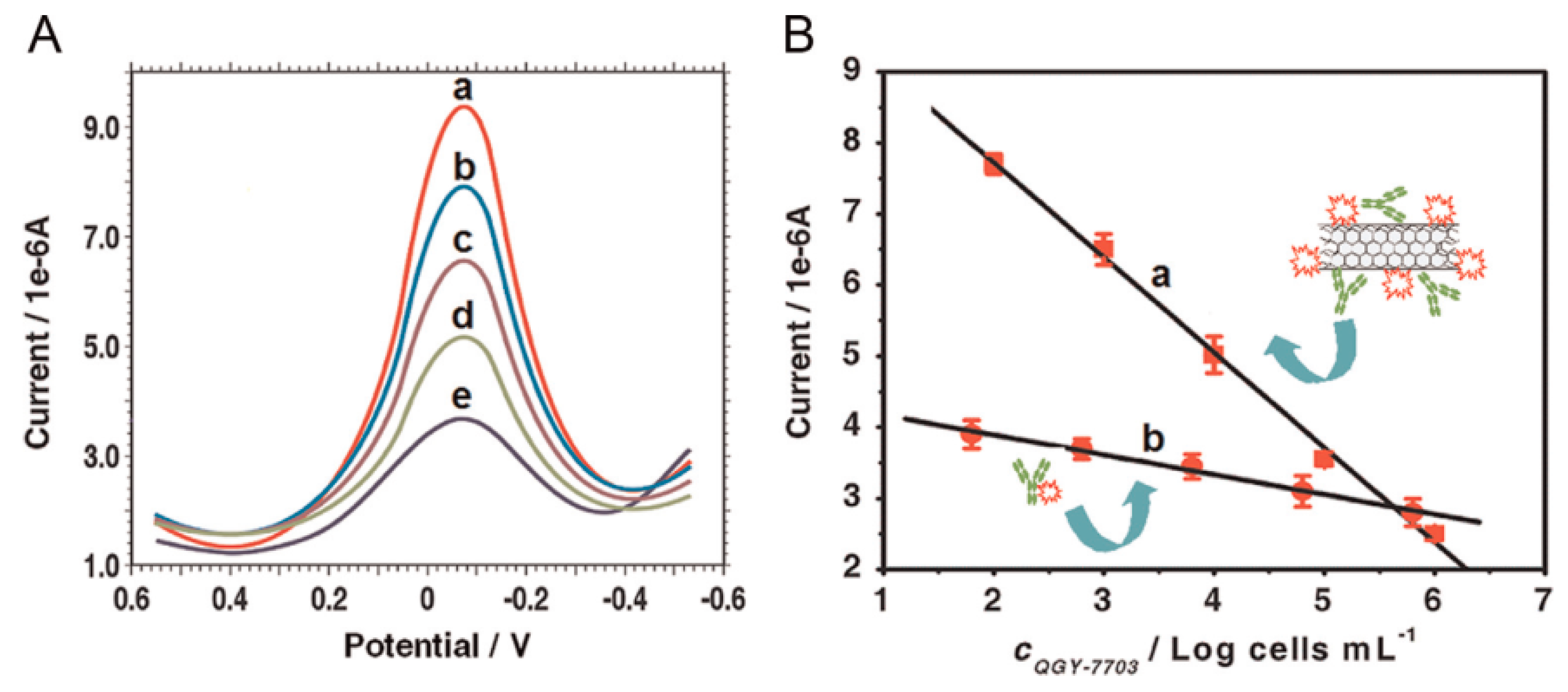
| Con A/MWCNTs | GCE | voltammetry | QGY-7703 | 100–100,000 cells mL−1 | 40 cells mL−1 | [86] |
| ferritin/MWCNTs | AuE | voltammetry | CA153 | 0.05–100 U mL−1 | 0.009 U mL−1 | [87] |
| HRP/MWCNTs | AuE | voltammetry | PSA | 0.001–10 ng mL−1 | 0.4 pg mL−1 | [88] |
| GOx/CNHs | GCE | voltammetry | AFP | 0.001–60 ng mL−1 | 0.33 pg mL−1 | [89] |
| nano Au/CNHs | SPE | voltammetry | AFP | 0.1–1000 pg mL−1 | 0.07 pg mL−1 | [90] |
| Nanomaterials Used | Electrode | Transduction Method | Analyte | Detection Range | LOD | Ref. |
|---|---|---|---|---|---|---|
| GO | SPGE | voltammetry | hCG | 5–500 pg mL−1 | 5 pg mL−1 | [95] |
| GO | SPGE | impedimetry | hCG | 0.001–50 ng mL−1 | 0.286 pg mL−1 | [98] |
| rGO | SPCE | voltammetry | AFP | 0.001–100 ng mL−1 | 1 pg mL−1 | [99] |
| rGO | SPCE | voltammetry | CEA | 0.005–100 ng mL−1 | 5 pg mL−1 | [99] |
| rGO | SPCE | voltammetry | CA125 | 0.001–100 ng mL−1 | 1 pg mL−1 | [99] |
| rGO | SPCE | voltammetry | CA153 | 0.005–100 ng mL−1 | 5 pg mL−1 | [99] |
| N-doped rGO | GCE | voltammetry | CA153 | 0.1–20 U mL−1 | 0.012 U mL−1 | [102] |
| multilayer GO | GO/SiC | impedimetry | hCG | 0.62–5.62 ng mL−1 | 0.62 ng mL−1 | [103] |
| graphene | graphene foam | voltammetry | CEA | 0.1–750 ng mL−1 | 90 pg mL−1 | [104] |
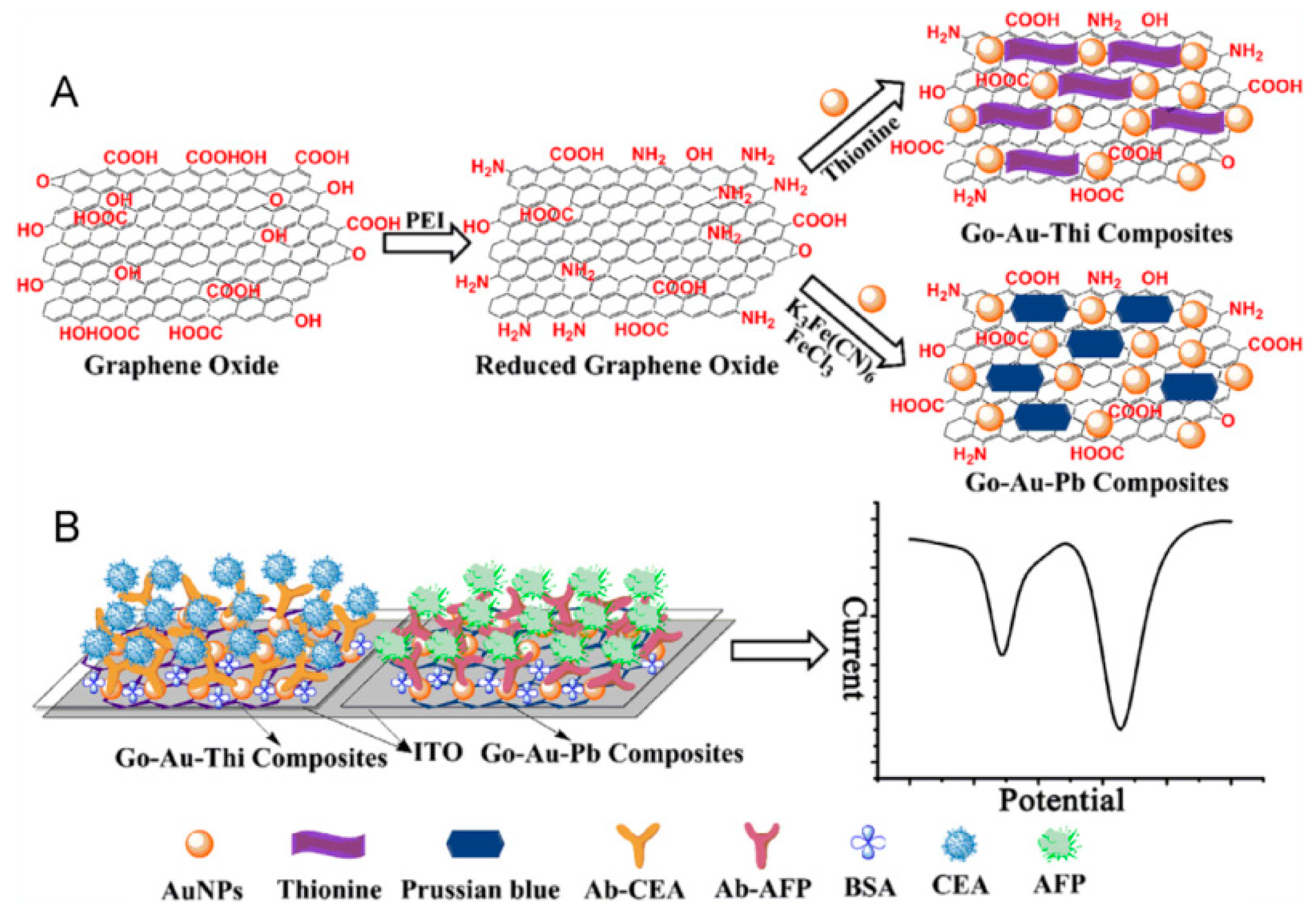
| thionine/GO | ITO | voltammetry | CEA | 0.01–300 ng mL−1 | 0.65 pg mL−1 | [105] |
| PB/GO | ITO | voltammetry | AFP | 0.01–300 ng mL−1 | 0.885 pg mL−1 | [105] |
| CoHCF/GO | GCE | voltammetry | PSA | 0.02–2 ng mL−1 | 0.01 ng mL−1 | [107] |
| ZrO2/rGO | ITO | voltammetry | CYFRA-21-1 | 2–22 ng mL−1 | 0.122 ng mL−1 | [108] |
| TB/GO-COOH | GCE | voltammetry | CEA | 0.5–60 ng mL−1 | 0.1 ng mL−1 | [111] |
| PB/GO-COOH | GCE | voltammetry | AFP | 0.5–60 ng mL−1 | 0.05 ng mL−1 | [111] |
| PB/AuNPs/GO | GCE | voltammetry | AFP | 0.01–100 ng mL−1 | 4.6 pg mL−1 | [112] |
| CuS/GO | SPCE | voltammetry | AFP | 0.001–10 ng mL−1 | 0.5 pg mL−1 | [113] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Akiba, U.; Anzai, J.-i. Recent Progress in Nanomaterial-Based Electrochemical Biosensors for Cancer Biomarkers: A Review. Molecules 2017, 22, 1048. https://doi.org/10.3390/molecules22071048
Wang B, Akiba U, Anzai J-i. Recent Progress in Nanomaterial-Based Electrochemical Biosensors for Cancer Biomarkers: A Review. Molecules. 2017; 22(7):1048. https://doi.org/10.3390/molecules22071048
Chicago/Turabian StyleWang, Baozhen, Uichi Akiba, and Jun-ichi Anzai. 2017. "Recent Progress in Nanomaterial-Based Electrochemical Biosensors for Cancer Biomarkers: A Review" Molecules 22, no. 7: 1048. https://doi.org/10.3390/molecules22071048





