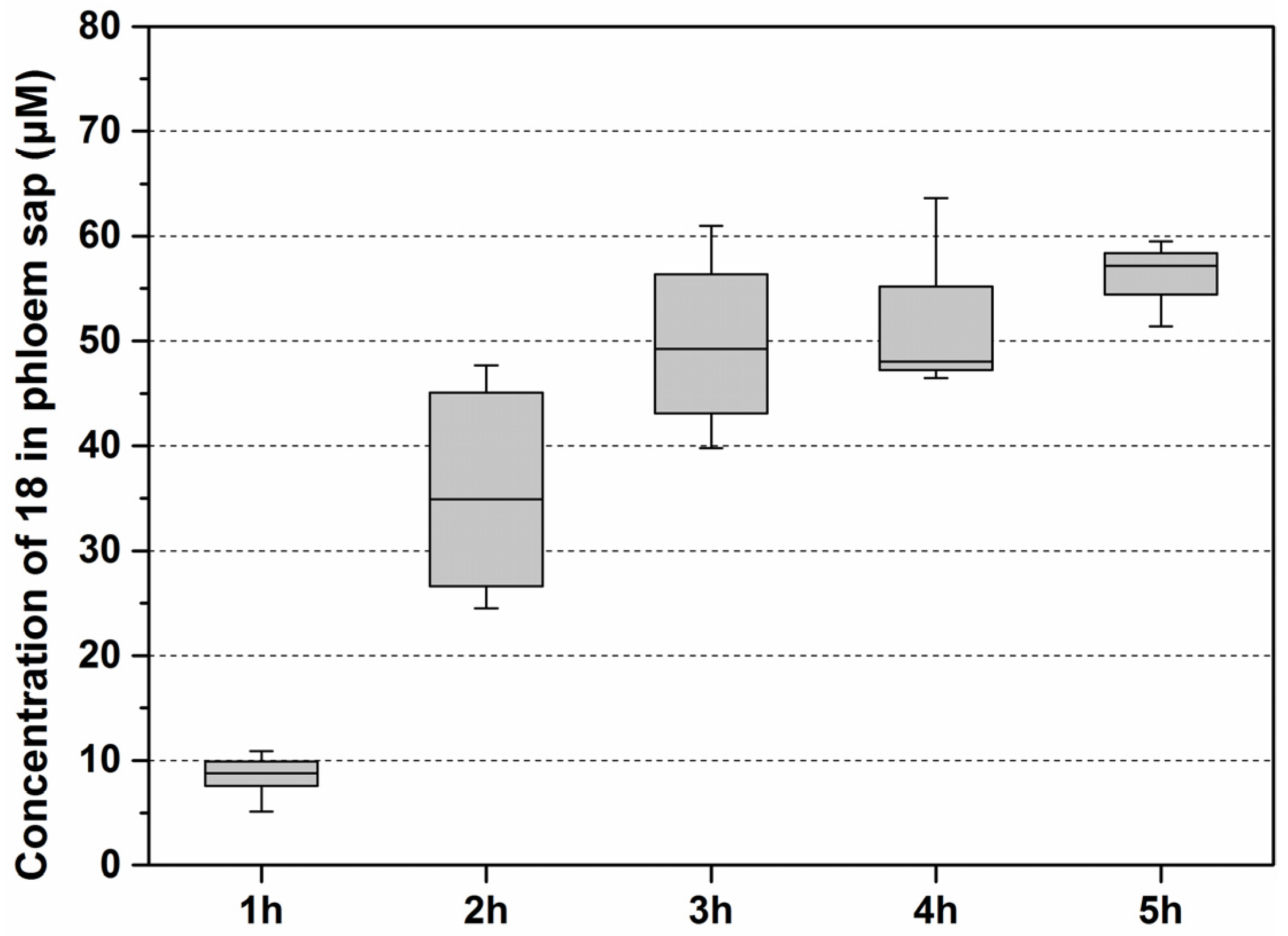
3.1. Synthesis
3.1.1. General Information
All reagents were purchased from a commercial company. Analytical thin-layer chromatography (TLC) was performed on silica gel GF254 plates (Qingdao Marine Chemical Ltd., Qingdao, China). Silica gel (100–200 and 200–300 mesh, Qingdao Marine Chemical Ltd., Qingdao, China) was used for column chromatography. 1H-and 13C-NMR spectra were obtained on a WNMR-I 500 (Wuhan Institute of Physics and Mathematics (WIPM) of Chinese Academy of Sciences, Wuhan, Hubei, China) or INOVA 400 (Agilent Technologies Inc., Santa Clara, CA, USA,) instrument. Chemical shifts were expressed in δ (ppm) values, with TMS as an internal standard. The mass spectra of new conjugates were obtained by high-performance liquid chromatography (HPLC)-mass spectrometry (MS) (Thermo, San Jose, CA, USA) using electrospray ionization (ESI) and are reported as m/z values.
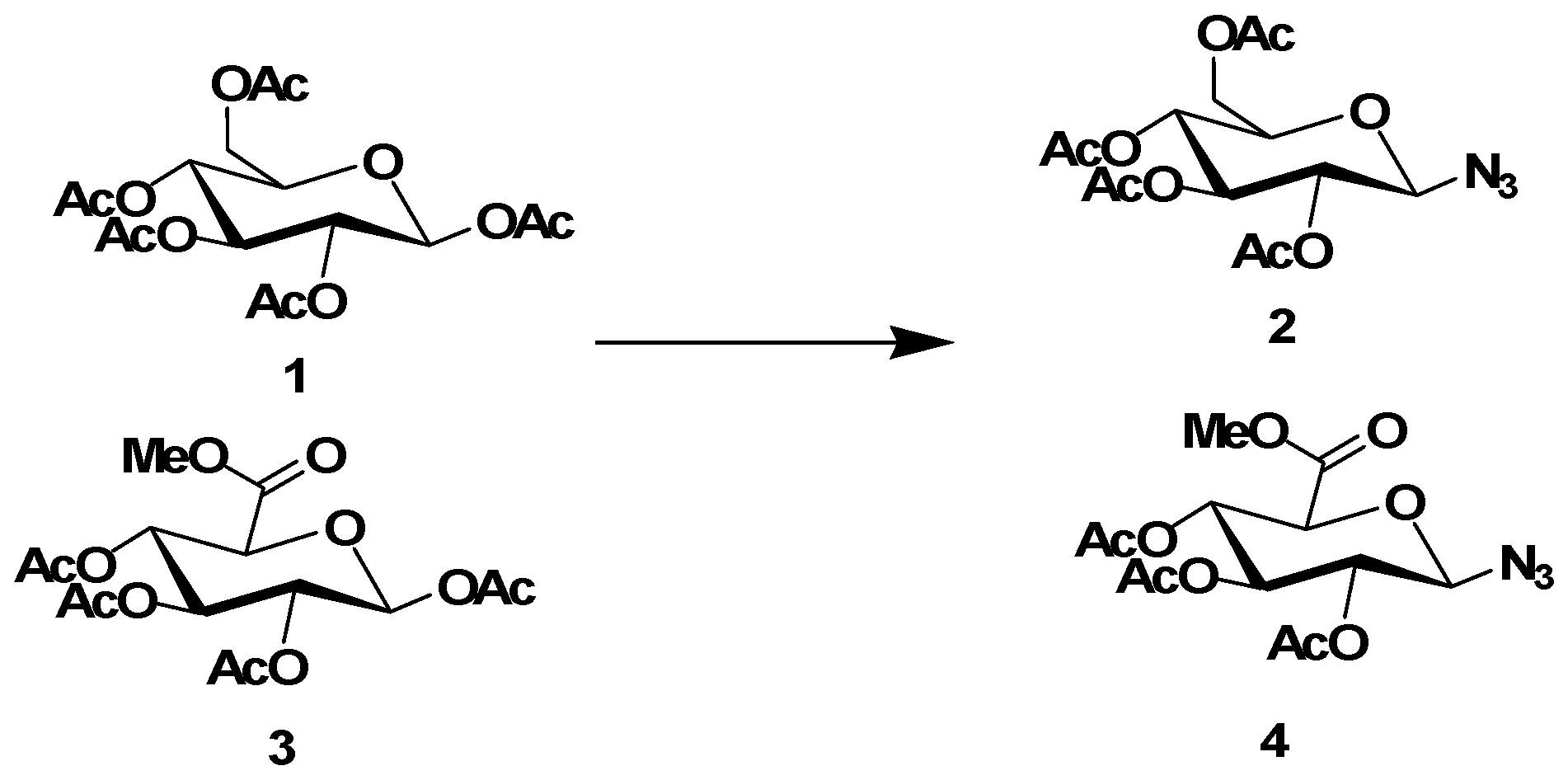
3.1.2. Synthesis of Azide Intermediates 2 and 4
Azide intermediates were synthesized according to previously reported procedures [
15,
16]. Briefly, compounds
1 or
3 (2 mmol) were added to a solution of azidotrimethylsilane (2.2 mmol) in anhydrous CH
2Cl
2 (10 mL), followed by dropwise addition of SnCl
4 (2 mmol). The resulting mixture was stirred at room temperature (rt) for 2 h, then extracted three times with aqueous sodium hydrogen carbonate and washed with brine, dried over sodium sulfate, filtered, and evaporated in vacuo. The residues were then purified byrecrystallization from ethanol to give the azide intermediates
2 or
4 as white solids.
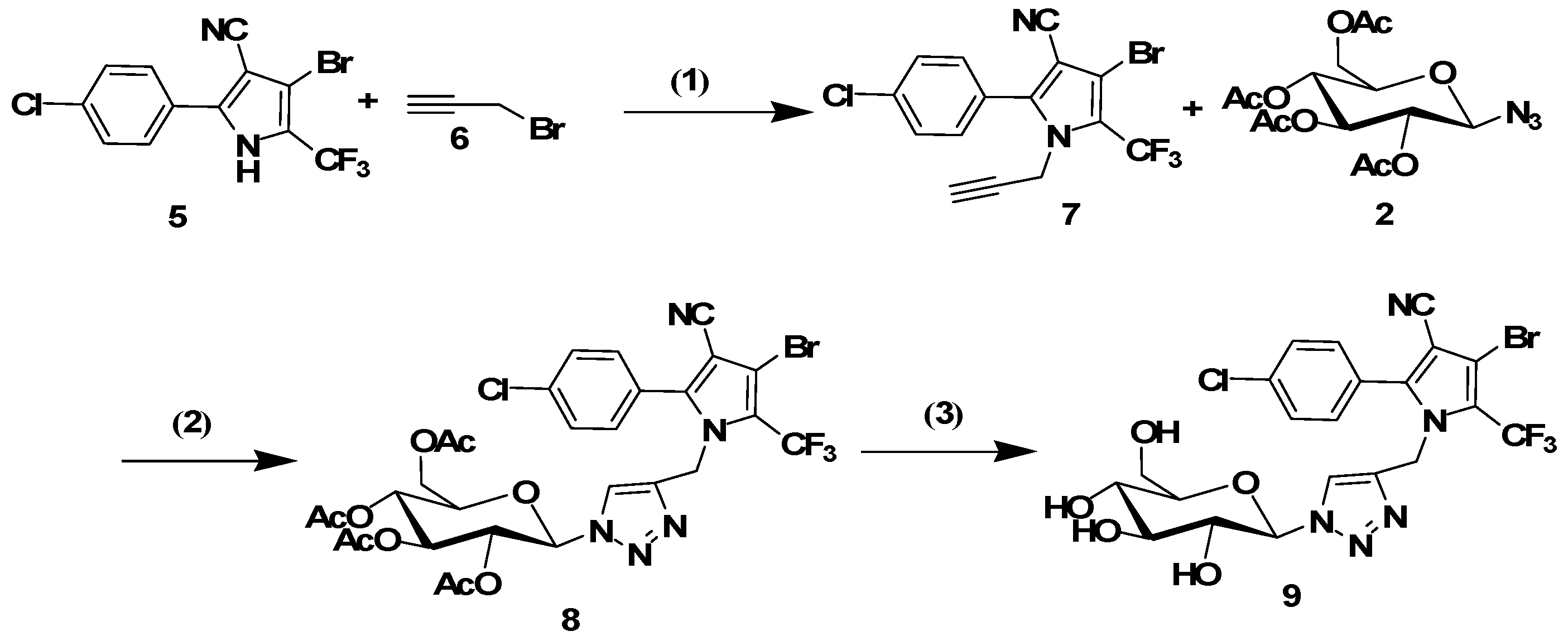
3.1.3. Synthesis of N-Propargyl Tralopyril (7)
Synthesis of the title compound was accomplished according to a previously reported procedure [
14]. Briefly, NaH (60% in oil, 0.144 g, 3.6 mmol) was added to the solution of tralopyril (1.05 g, 3 mmol) in THF (15 mL). The mixture was then stirred at room temperature for 2 h. Propargyl bromide (3.3 mmol) in THF (15 mL) was dropwise added at room temperature. The mixture was then stirred at 65 °C for 48 h. The reaction mixture was quenched by adding ice-water, and the resulting mixture was extracted with ethyl acetate (15 mL × 3). The combined organic layers were washed with aqueous sodium hydrogen carbonate and brine, dried with sodium sulfate, filtered, and evaporated in vacuo. The residue was purified by column chromatography (10 mL min
−1) to afford the desired product
7.
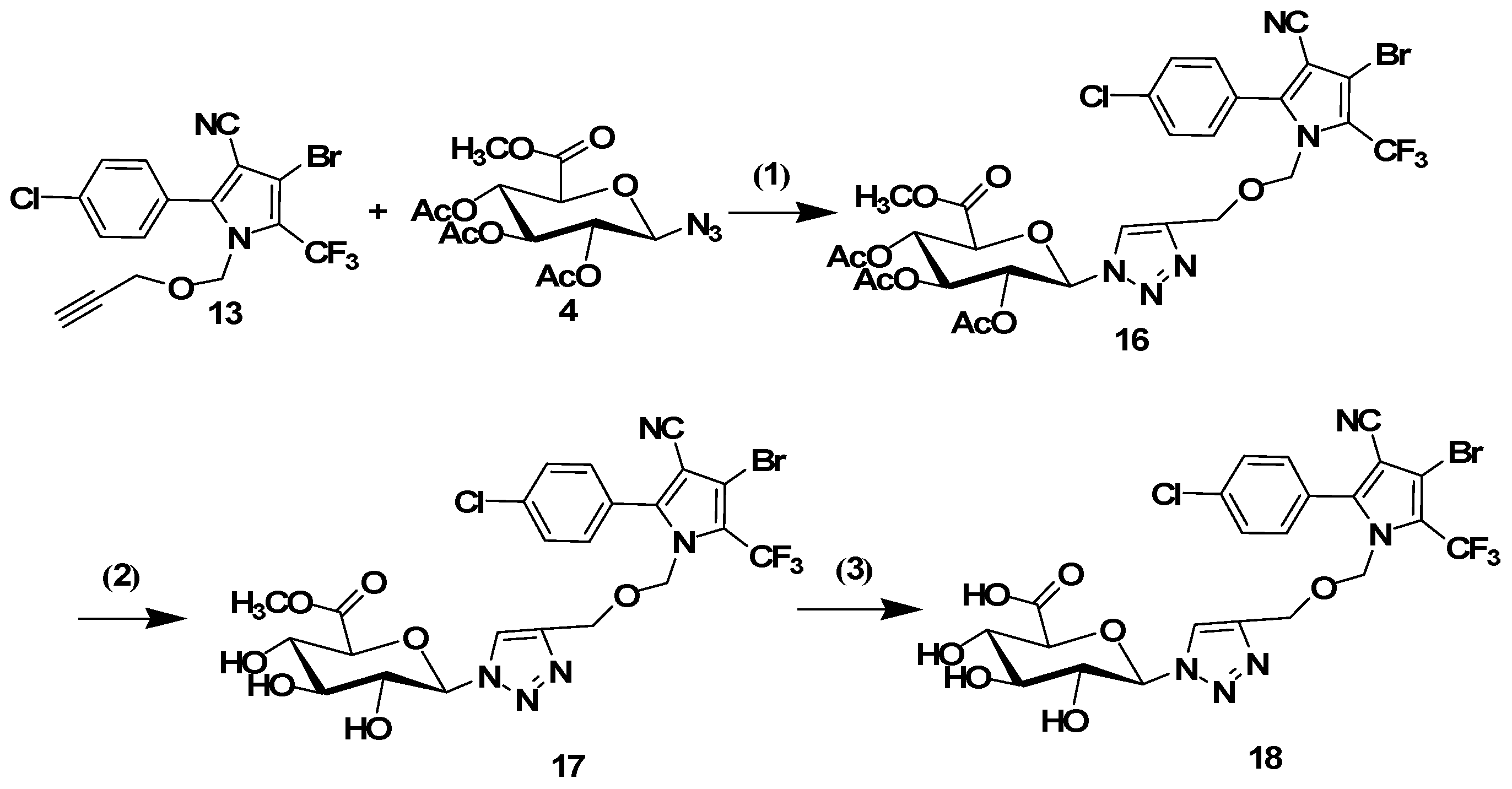
3.1.4. Synthesis of N-Propynoxymethyl Tralopyril (13)
The title compound was synthesized according to a previously reported procedure [
14]. Briefly, NaH (60% in oil, 0.288 g, 7.2 mmol) was added to the solution of tralopyril (1.05 g, 3 mmol) in mixture solvent of THF (20 mL) and bromochloromethane (1.95 g, 15 mmol, 5 equiv). The mixture was then stirred at room temperature for 2 h. Propargyl alcohol (3 mmol) in THF (10 mL) was dropwise added at 65 °C. The mixture was then stirred at 65 °C for 48 h. The reaction mixture was quenched by adding ice-water, and the resultant mixture was extracted with ethyl acetate (20 mL × 3). The combined organic layers were washed with aqueous sodium hydrogen carbonate and brine, dried with sodium sulfate, filtered, and evaporated in vacuo. The residue was purified by column chromatography (10 mL min
−1) to give the desired product
13.
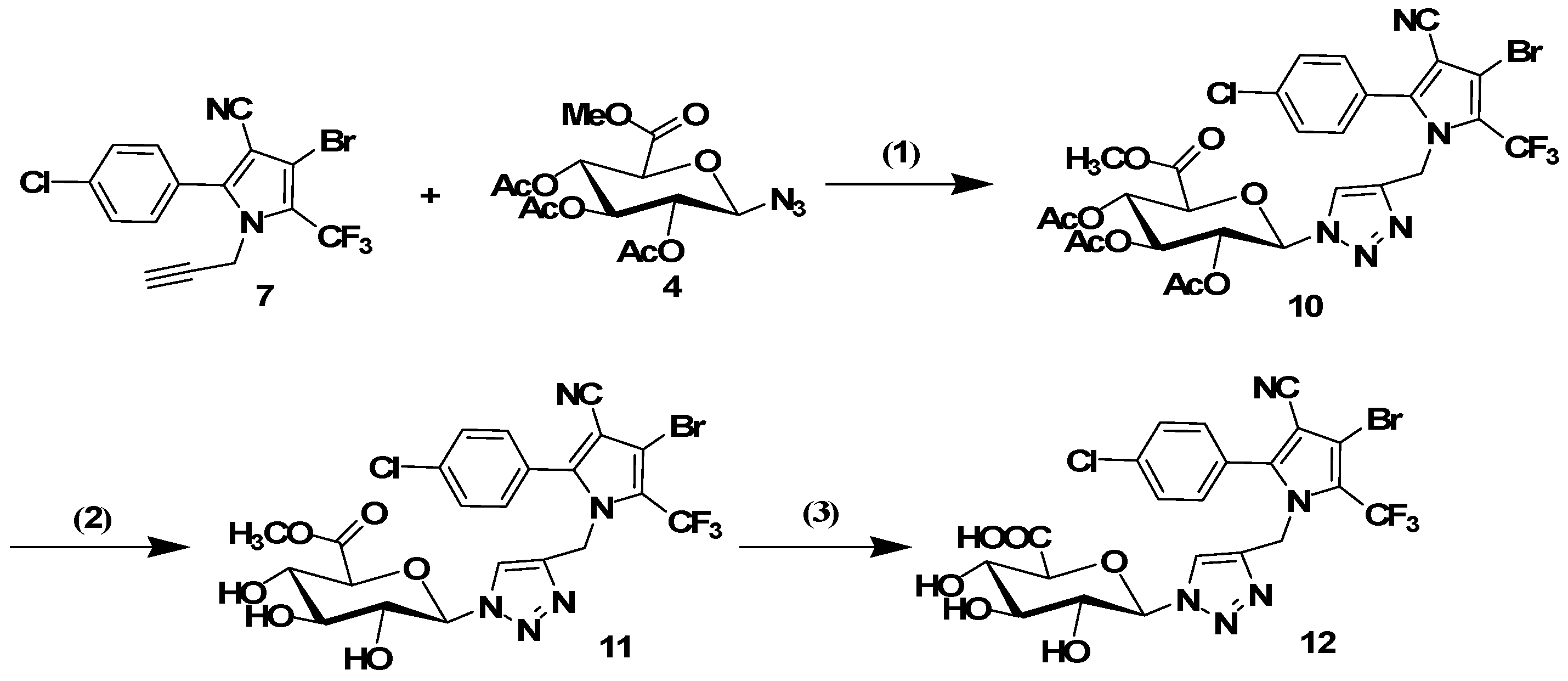
3.1.5. General Procedure for the Click Reaction
The conjugates 8, 10, 14 and 16 were synthesized using the following procedure: tralopyril terminal alkyne derivatives (compounds 7 or 13, 1 mmol) and an azidoglycose (compounds 2 or 4, 1 mmol) were dissolved in t-BuOH (3 mL). The reaction was initiated by the addition of CuSO4·5H2O (0.2 mmol) and sodium ascorbate (0.4 mmol) in distilled water (3 mL). The mixture was stirred at 60 °C until TLC indicated the disappearance of the starting materials. The mixture was poured into distilled water (20 mL), and the product was extracted three times with CH2Cl2 (20 mL). The organic layer was dried with Na2SO4 and filtered. The solvent was removed under reduced pressure. The residues were purified by column chromatography (1:2 petroleum ether/ethyl acetate) to obtain the glycosyl tralopyril conjugates 8, 10, 14 and 16.
4-Bromo-2-(4-chlorophenyl)-1-((1-(2,3,4,6-tetra-O-acetyl-beta-d-glucopyranosyl)-1H-1,2,3-triazol-4-yl)methyl)-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile (8). 1H-NMR (500 MHz, DMSO-d6) δ 8.31 (s, 1H), 7.66–7.59 (m, 4H), 6.31 (d, J = 9.2 Hz, 1H), 5.62 (t, J = 9.4 Hz, 1H), 5.53 (t, J = 9.5 Hz, 1H), 5.28 (s, 2H), 5.17 (t, J = 9.8 Hz, 1H), 4.34 (m, 1H), 4.16–4.05 (m, 2H), 2.02 (s, 3H), 2.00 (s, 3H), 1.96 (s, 3H), 1.77 (s, 3H). 13C-NMR (126 MHz, DMSO-d6) δ 170.04, 169.59, 169.42, 168.42, 144.43, 142.72, 135.87, 131.80, 129.33, 125.50, 122.08, 113.69, 102.68, 97.92, 83.86, 73.24, 72.10, 70.13, 67.43, 61.59, 43.30, 20.52, 20.42, 20.26, 19.81. ESI-MS m/z: 761 [M + 1]+, 783 [M + Na]+.
2-(4-((3-Bromo-5-(4-chlorophenyl)-4-cyano-2-(trifluoromethyl)-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10). 1H-NMR (400 MHz, DMSO-d6) δ 8.31 (s, 1H), 7.62 (m, 4H), 6.30 (d, J = 9.1 Hz, 1H), 5.62 (t, J = 9.3 Hz, 1H), 5.52 (t, J = 9.5 Hz, 1H), 5.28 (s, 2H), 5.17 (t, J = 9.7 Hz, 1H), 4.33 (m, 1H), 3.65 (s, 3H), 2.02 (s, 3H), 2.00 (s, 3H), 1.96 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 170.07, 169.61, 169.44, 168.45, 144.44, 142.73, 135.89, 131.82, 129.34, 125.51, 122.10, 113.71, 83.87, 73.26, 72.12, 70.14, 67.44, 61.60, 52.10, 43.31, 20.55, 20.45, 20.29. ESI-MS m/z: 748 [M + 1]+, 770 [M + Na]+.
4-Bromo-2-(4-chlorophenyl)-5-(trifluoromethyl)-1-((1-(2,3,4,6-tetra-O-acetyl-beta-d-glucopyranosyl-1H-1,2,3-triazol-4-yl)methoxy)methyl)-1H-pyrrole-3-carbonitrile (14). 1H-NMR (400 MHz, DMSO-d6) δ 8.26 (s, 1H), 7.67 (d, J = 1.1 Hz, 4H), 6.33 (d, J = 8.7 Hz, 1H), 5.66–5.51 (m, 2H), 5.36 (m, 2H), 5.19 (t, J = 9.5 Hz, 1H), 4.42 (s, 2H), 4.37 (m, 1H), 4.18–4.03 (m, 2H), 2.03 (s, 3H), 2.00 (s, 3H), 1.97 (s, 3H), 1.76 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 170.08, 169.62, 169.44, 168.52, 144.53, 143.05, 135.90, 131.97, 129.35, 125.35, 123.29, 113.63, 98.01, 83.86, 75.44, 73.29, 72.12, 70.16, 67.53, 61.85, 61.16, 20.55, 20.44, 20.30, 19.90. ESI-MS m/z: 792 [M + 1]+, 814 [M + Na]+.
2-(4-(((3-Bromo-5-(4-chlorophenyl)-4-cyano-2-(trifluoromethyl)-1H-pyrrol-1-yl)methoxy)methyl)-1H-1,2,3-triazol-1-yl)-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (16). 1H-NMR (500 MHz, DMSO-d6) δ 8.25 (s, 1H), 7.71–7.63 (m, 4H), 6.33 (d, J = 8.8 Hz, 1H), 5.65–5.50 (m, 2H), 5.40–5.32 (m, 2H), 5.19 (t, J = 9.6 Hz, 1H), 4.42 (s, 2H), 4.36 (m, 1H), 3.70 (s, 3H),2.03 (s, 3H), 2.00 (s, 3H), 1.97 (s, 3H).13C-NMR (126 MHz, DMSO-d6) δ 170.09, 169.63, 169.44, 168.53, 144.53, 143.06, 135.90, 131.97, 129.35, 125.36, 123.29, 113.63, 103.29, 98.02, 83.86, 75.43, 73.29, 72.12, 70.16, 67.53, 61.84, 61.16, 52.11, 20.54, 20.43, 20.29. ESI-MS m/z: 778 [M + 1]+, 800 [M + Na]+.
3.1.6. General Procedure for the Removal of Acetyl Groups
The conjugates 8, 10, 14 or 16 (1 mmol) were added to a solution of sodium methoxide in dry methanol (0.05 M, 15 mL). The resulting solution was stirred for 30 min at room temperature. The mixture was neutralized with Amberlite IR120 (H+) resin and filtered. Subsequently, the filtrate was evaporated. The residues were purified by column chromatography (9:1 CH2Cl2/MeOH) to afford the glycosyl tralopyril conjugate 9, 11, 15 or 17.
4-Bromo-2-(4-chlorophenyl)-1-((1-(beta-d-glucopyranosyl)-1H-1,2,3-triazol-4-yl)methyl)-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile (9). 1H-NMR (400 MHz, DMSO-d6): δ 7.94 (s, 1H), 7.62 (m, 4H), 5.45 (d, J = 9.4 Hz, 1H), 5.27 (s, 2H), 3.90–3.88 (m, 2H), 3.70–3.65 (m, 3H), 3.50–3.48 (m, 1H).13C-NMR (101 MHz, DMSO-d6) δ 144.4, 141.6, 135.7, 131.9, 129.3, 127.1, 125.6, 122.3, 121.1, 113.7, 102.5, 97.9, 87.5, 80.0, 76.9, 72.0, 70.0, 60.7, 43.2. ESI-MS m/z: 594 [M + 1]+, 617 [M + Na]+.
Methyl6-(4-((3-bromo-5-(4-chlorophenyl)-4-cyano-2-(trifluoromethyl)-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylate (11). 1H NMR (400 MHz, MeOD) δ 7.89 (s, 1H), 7.48 (m, 4H), 5.59 (d, J = 9.3 Hz, 1H), 5.34 (s, 2H), 4.11 (d, J = 9.8 Hz, 1H), 3.89 (t, J = 9.2 Hz, 1H), 3.74 (s, 3H), 3.66 (t, J = 9.5 Hz, 1H), 3.53 (t, J = 9.1 Hz, 1H). 13C-NMR (101 MHz, MeOD) δ 170.49, 146.03, 143.76, 138.11, 132.83, 130.51, 127.10, 123.53, 122.96, 121.11, 120.24, 114.26, 103.51, 101.12, 89.14, 79.07, 77.80, 73.30, 72.66, 53.02, 44.17. ESI-MS m/z: 622 [M + 1]+, 644 [M + Na]+.
4-Bromo-2-(4-chlorophenyl)-5-(trifluoromethyl)-1-((1-(beta-d-glucopyranosyl-1H-1,2,3-triazol-4-yl)methoxy)methyl)-1H-pyrrole-3-carbonitrile (15). 1H-NMR (400 MHz, DMSO-d6) δ 8.11 (s, 1H), 7.74–7.62 (m, 4H), 5.50 (d, J = 9.2 Hz, 1H), 5.40 (s, 2H), 5.38 (d, J = 5.9 Hz, 1H), 5.31 (d, J = 4.9 Hz, 1H), 5.18 (d, J = 5.5 Hz, 1H), 4.64 (t, J = 5.5 Hz, 1H), 4.39 (s, 2H), 3.69 (m, 2H). 13C-NMR (101 MHz, DMSO-d6) δ 144.55, 142.23, 135.97, 131.99, 129.45, 125.37, 123.54, 113.68, 98.01, 87.49, 80.01, 76.95, 75.58, 72.14, 69.59, 61.36, 60.80. ESI-MS m/z: 624 [M + 1]+, 646 [M + Na]+.
Methyl 6-(4-(((3-bromo-5-(4-chlorophenyl)-4-cyano-2-(trifluoromethyl)-1H-pyrrol-1-yl)methoxy)methyl)-1H-1,2,3-triazol-1-yl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylate (17). 1H-NMR (400 MHz, DMSO-d6) δ 8.18 (s, 1H), 7.70–7.57 (m, 4H), 5.71 (d, J = 9.3 Hz, 1H), 5.57 (m, 2H), 5.39 (s, 2H), 4.41 (s, 2H), 4.15 (d, J = 9.6 Hz, 1H), 3.82 (m, 1H), 3.66 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 168.78, 144.52, 142.39, 135.93, 131.91, 129.37, 125.31, 123.71, 113.66, 103.33, 87.07, 77.46, 76.10, 75.50, 71.61, 71.19, 61.25, 52.12. ESI-MS m/z: 652 [M + 1]+, 674 [M + Na]+.
3.1.7. General Procedure for the Removal of Methyl Ester Groups
KOH (1.3mmol) was added to a solution of 11 or 17 (1 mmol) in MeOH (8 mL) at 0 °C. The reaction mixture was stirred overnight at room temperature and acidified via treatment with Amberlite IR 120H+. Filtration, evaporation under reduced pressure, and purification afforded 12 or 18 as a white solid.
6-(4-((3-Bromo-5-(4-chlorophenyl)-4-cyano-2-(trifluoromethyl)-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic acid (12). 1H-NMR (400 MHz, MeOD) δ 7.91 (s, 1H, Ar-H), 7.50 (q, J = 8.6 Hz, 4H, Ar-H), 5.60 (d, J = 9.3 Hz, 1H, H-1), 5.36 (s, 2H , NCH2), 4.07 (d, J = 9.7 Hz, 1H, H-5), 3.89 (t, J = 9.1 Hz, 1H, H-3), 3.67 (t, J = 9.4 Hz, 1H, H-4), 3.55 (t, J = 9.1 Hz, 1H, H-2). 13C-NMR (101 MHz, MeOD) δ 171.75, 146.05, 143.80, 138.13, 132.83, 130.51, 130.25, 127.09, 123.48, 122.97, 121.14, 120.29, 114.26, 103.47, 100.14, 89.16, 78.99, 77.96, 73.38, 72.70, 44.21. ESI-MS m/z: 608 [M + 1]+, 630 [M + Na]+.
6-(4-(((3-Bromo-5-(4-chlorophenyl)-4-cyano-2-(trifluoromethyl)-1H-pyrrol-1-yl)methoxy)methyl)-1H-1,2,3-triazol-1-yl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic acid (18). 1H-NMR (500 MHz, DMSO-d6) δ 8.17 (s, 1H), 7.71–7.60 (m, 4H), 5.65 (d, J = 9.3 Hz, 1H), 5.39 (s, 2H), 4.41 (m, 2H), 3.99 (d, J = 9.7 Hz, 1H), 3.79 (t, J = 9.1 Hz, 1H), 3.50 (t, J = 9.3 Hz, 1H), 3.43 (t, J = 8.9 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6) δ 169.70, 144.54, 142.35, 135.96, 131.91, 129.39, 125.32, 123.72, 113.66, 103.31, 97.97, 87.14, 77.80, 76.32, 75.49, 71.70, 71.21, 61.26. ESI-MS m/z: 638 [M + 1]+, 660 [M + Na]+.