Effects on Rotational Dynamics of Azo and Hydrazodicarboxamide-Based Rotaxanes †
Abstract
:1. Introduction
2. Results
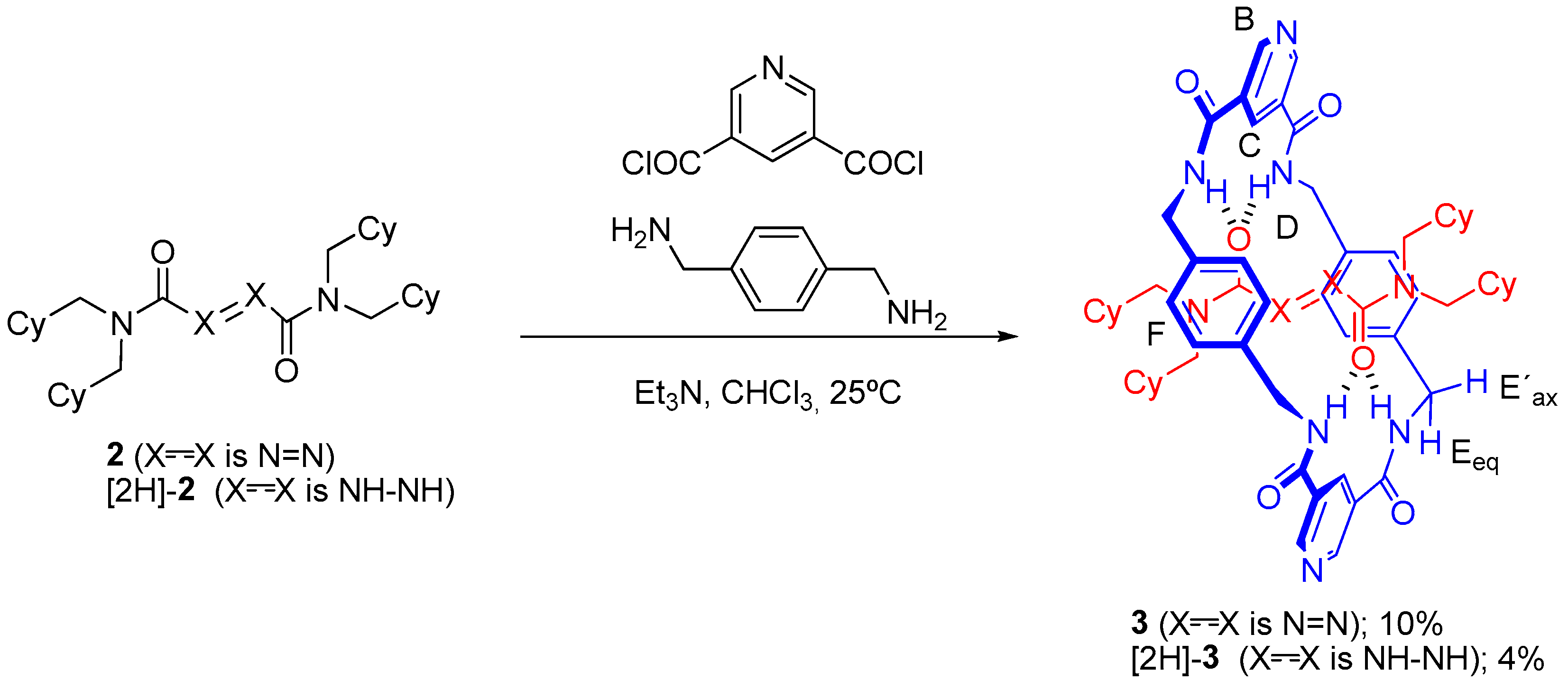
2.1. Synthesis of Threads [2H]-2 and 2
2.2. Synthesis of Rotaxanes [2H]-3 and 3
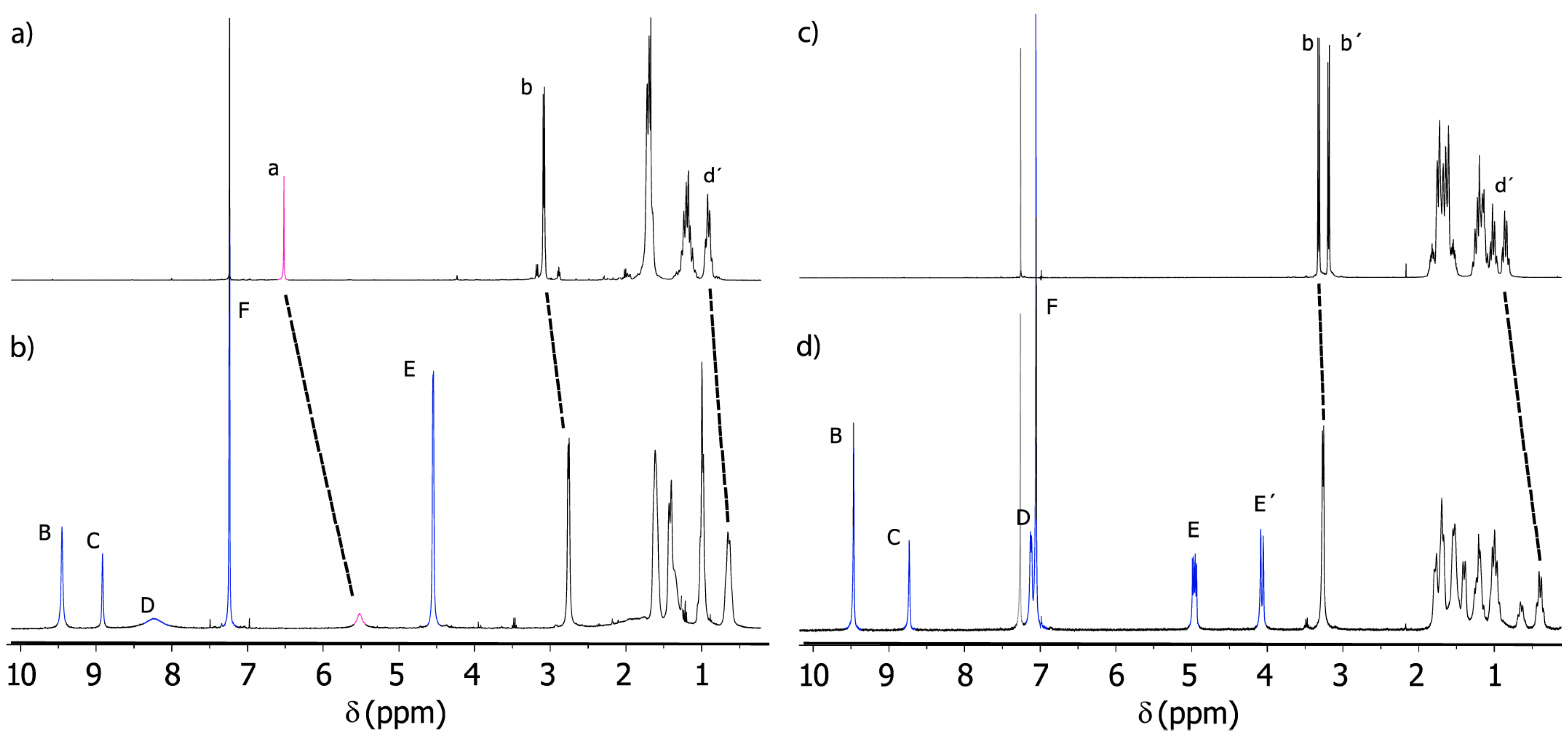
2.3. Analysis of Threads 2 and Rotaxanes 3
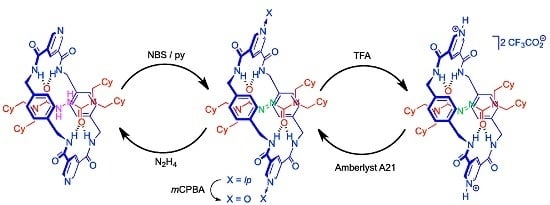
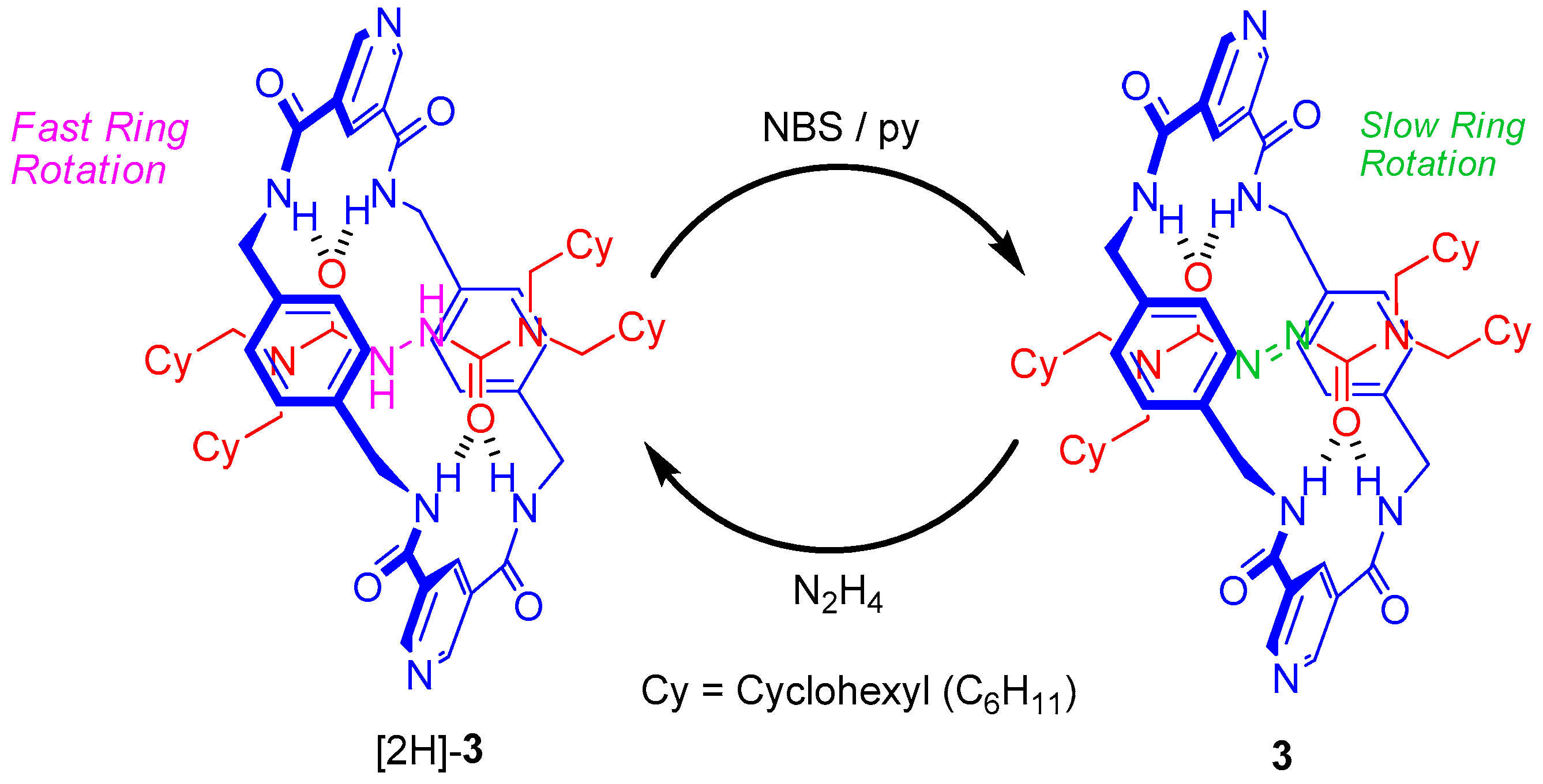
2.4. Chemical Interconversion between Rotaxanes 3
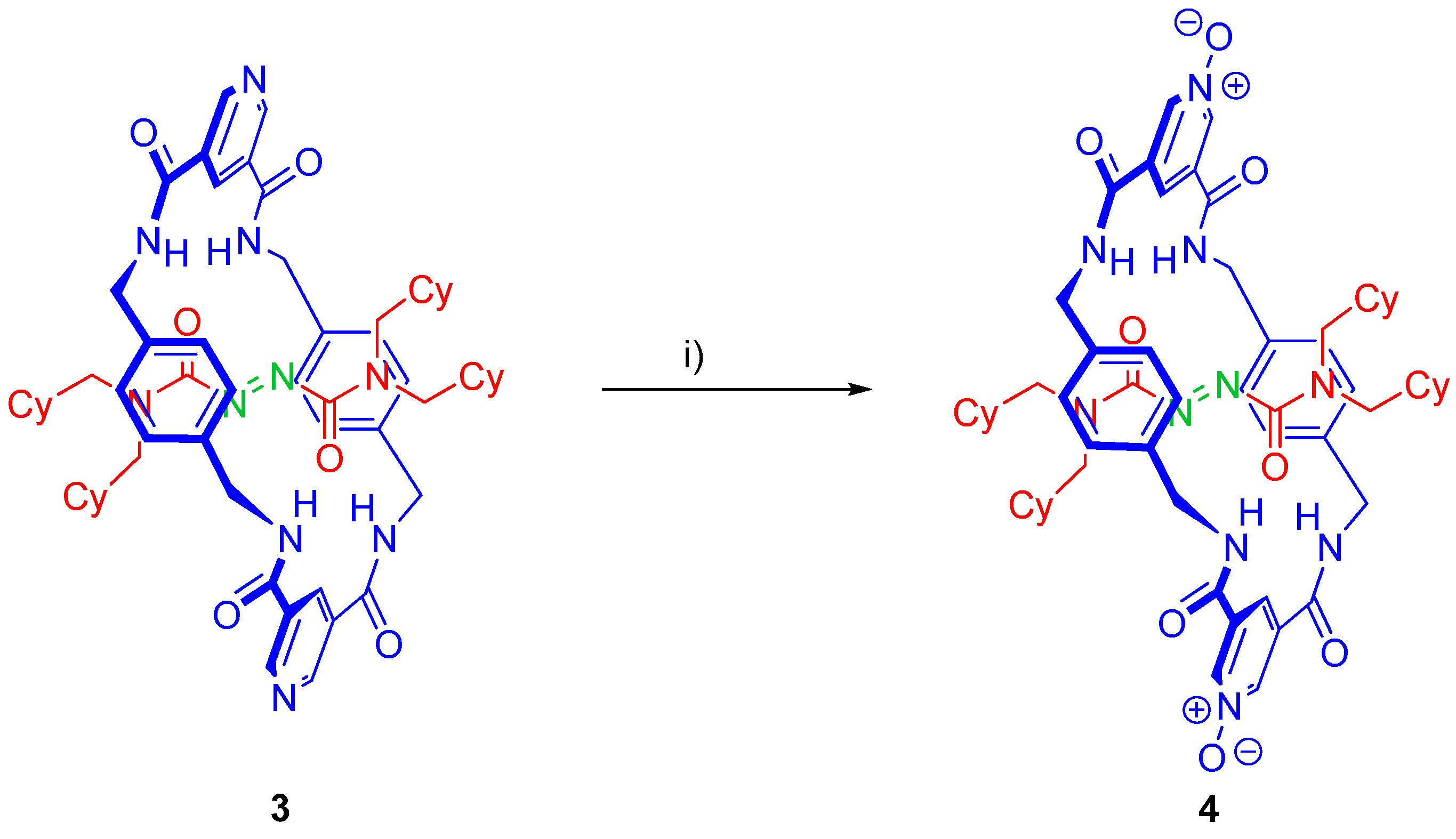
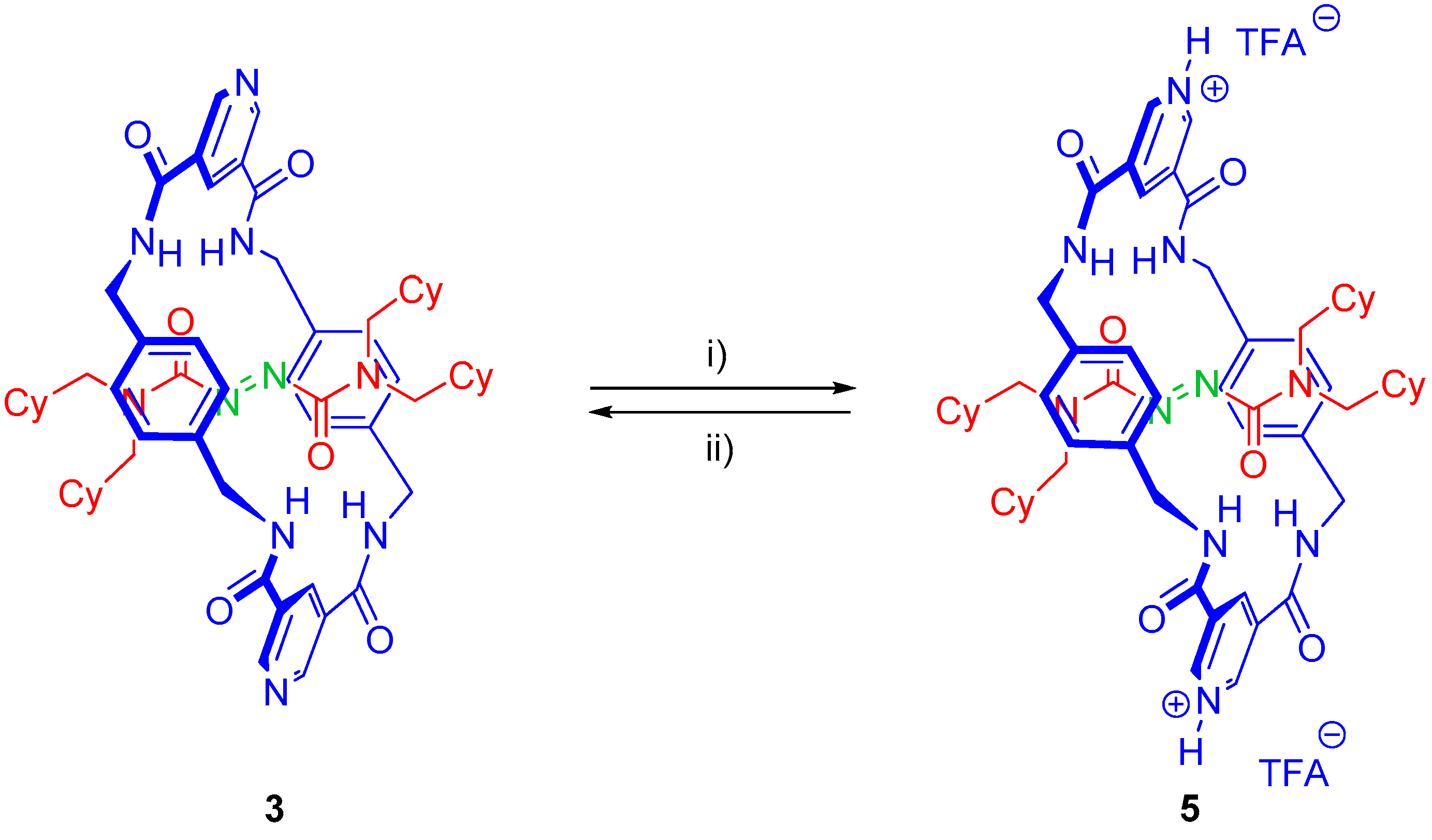
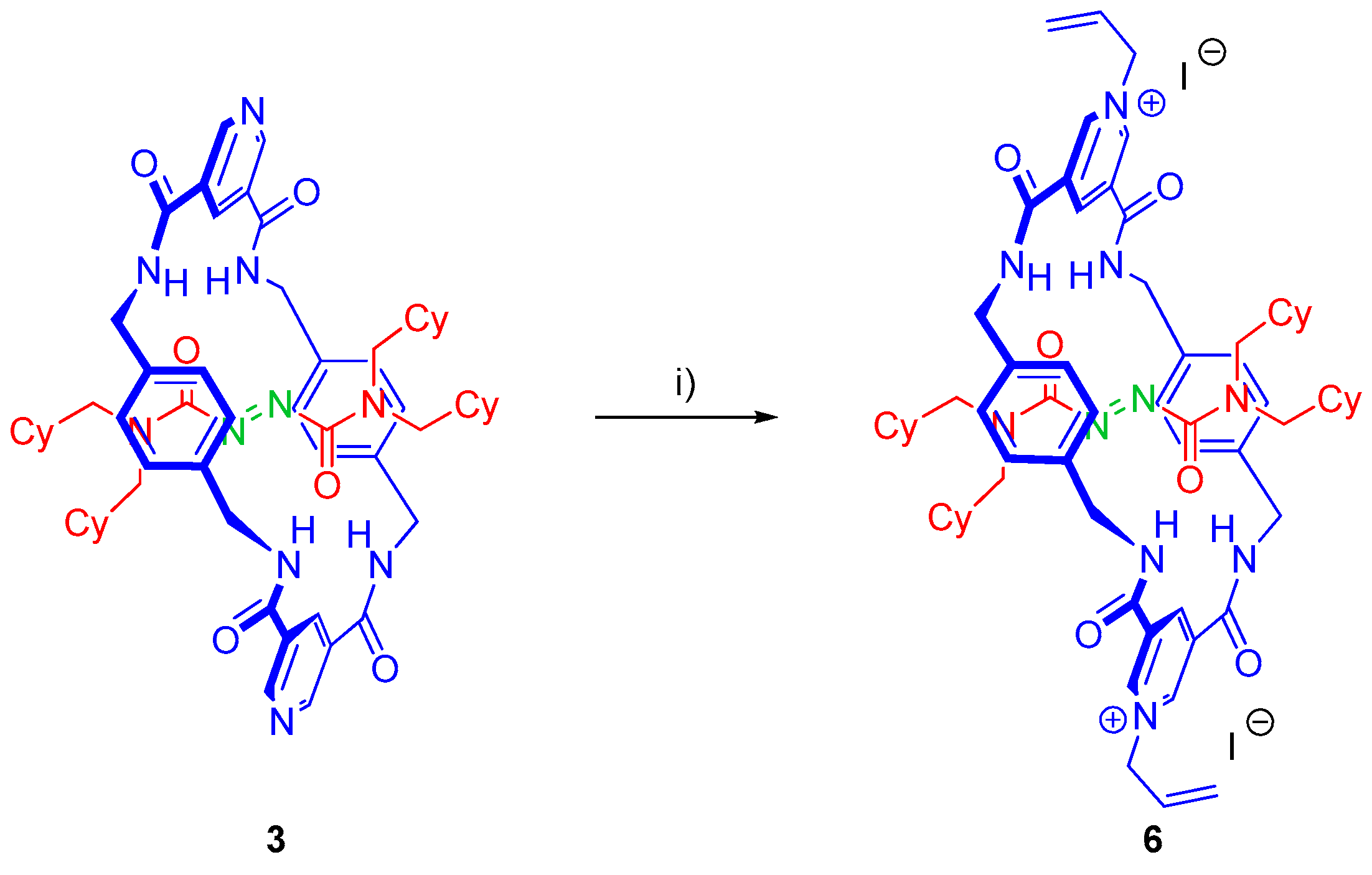
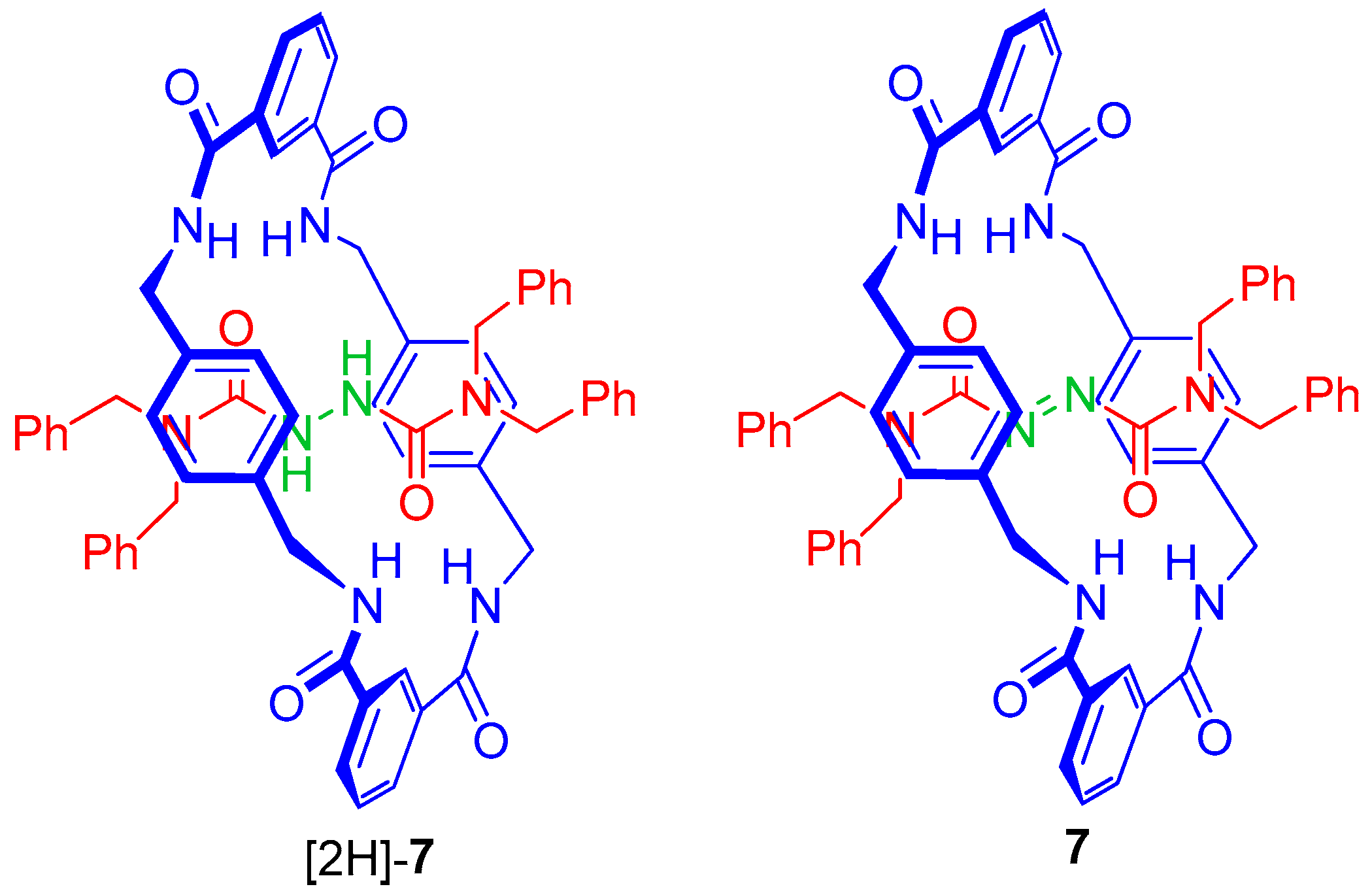
2.5. Chemical Functionalization of the Pyridine Rings of the Macrocycle of Rotaxane 3
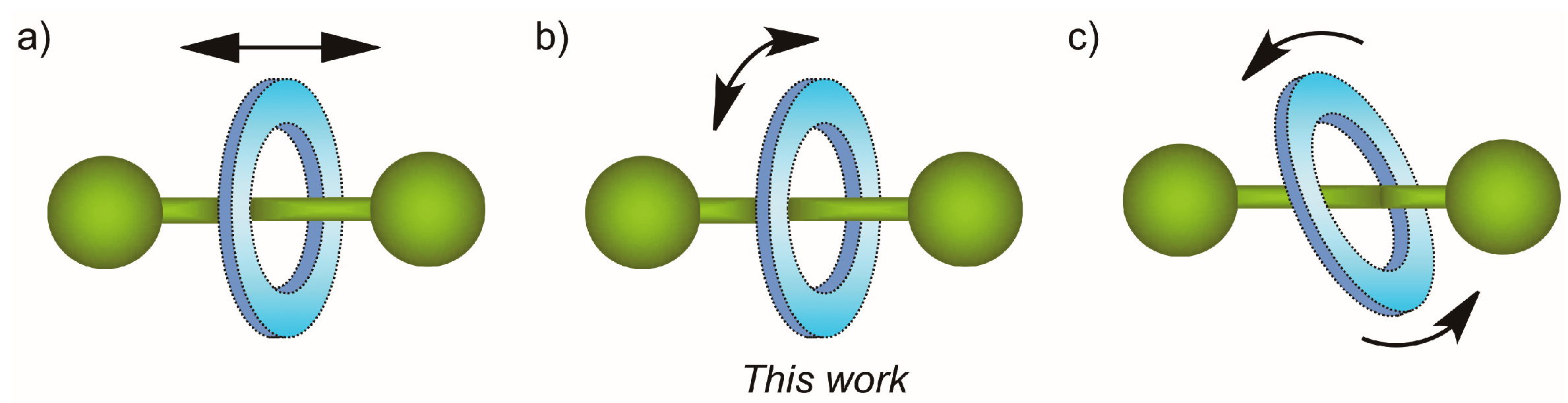
2.6. Variable-Temperature 1H-NMR Experiments: Calculation of the Rotational Barrier
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Threads
3.2.1. Synthesis of N1,N1,N2,N2-Tetrakis(cyclohexylmethyl)-1,2-hydrazodicarboxamide ([2H]-2)
3.2.2. Synthesis of N1,N1,N2,N2-Tetrakis(cyclohexylmethyl)-1,2-azodicarboxamide (2)
3.3. Synthesis of Rotaxanes
3.3.1. General Procedure for the Preparation of [2]Rotaxanes 3 and [2H]-3
3.4. Chemical Exchange of [2]Rotaxanes 3 and [2H]-3
3.4.1. Reduction Protocol
3.4.2. Oxidation Protocol
3.5. Synthesis of Rotaxane 4
3.6. Synthesis of Rotaxane 5
3.7. Synthesis of Rotaxane 6
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dietrich-Buchecker, C.; Sauvage, J. Interlocking of molecular threads: From the statistical approach to the templated synthesis catenands. Chem. Rev. 1987, 87, 795–810. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Stoddart, J.F. Interlocked and intertwined structures and superstructures. Chem. Rev. 1995, 95, 2725–2829. [Google Scholar] [CrossRef]
- Beves, J.E.; Blight, B.A.; Campbell, C.J.; Leigh, D.A.; McBurney, R.T. Strategies and tactics for the metal-directed synthesis of rotaxanes, knots, catenanes, and higher order links. Angew. Chem. Int. Ed. 2011, 50, 9260–9327. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Development of Pseudorotaxanes and Rotaxanes: From Synthesis to Stimuli-Responsive Motions to Applications. Chem. Rev. 2015, 115, 7398–7501. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ramirez, G.; Leigh, D.A.; Stephens, J.A. Catenanes: Fifty years of molecular links. Angew. Chem. Int. Ed. 2015, 54, 6110–6150. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, J.-P. Transition metal-containing rotaxanes and catenanes in motions: Toward molecular machines and motors. Acc. Chem. Res. 1998, 31, 611–619. [Google Scholar] [CrossRef]
- Balzani, V.; Credi, A.; Raymo, F.M.; Stoddart, J.F. Artificial Molecular Machines. Angew. Chem. Int. Ed. 2000, 39, 3348–3391. [Google Scholar] [CrossRef]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef] [PubMed]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial molecular machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, Y.; Wang, Y.; Liu, Z.; Cheng, C.; Hartlieb, K.J.; Wasielewski, M.R.; Stoddart, J.F. An Electrochromic Tristable Molecular Switch. J. Am. Chem. Soc. 2015, 137, 13484–13487. [Google Scholar] [CrossRef] [PubMed]
- Vukotic, N.; O’Keefe, C.A.; Zhu, K.; Harris, K.J.; To, C.; Schurko, R.W.; Loeb, S.J. Mechanically Interlocked Linkers inside Metal–Organic Frameworks: Effect of Ring Size on Rotational Dynamics. J. Am. Chem. Soc. 2015, 137, 9643–9651. [Google Scholar] [CrossRef] [PubMed]
- Barendt, T.A.; Ferreira, L.; Marques, I.; Felix, V.; Beer, P.D. Anion- and solvent-induced rotary dynamics and sensing in a perylene diimide [3]catenane. J. Am. Chem. Soc. 2017. [Google Scholar] [CrossRef] [PubMed]
- Berna, J.; Bottari, G.; Leigh, D.A.; Perez, E.M. Amide-based molecular shuttles (2001–2006). Pure Appl. Chem. 2007, 79, 39–54. [Google Scholar] [CrossRef]
- Panman, M.R.; Bakker, B.H.; den Uyl, D.; Kay, E.R.; Leigh, D.A.; Buma, W.J.; Brouwer, A.M.; Geenevasen, J.A.J.; Woutersen, S. Water lubricates hydrogen-bonded molecular machines. Nat. Chem. 2013, 5, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cuezva, A.; Lopez-Leonardo, C.; Bautista, D.; Alajarin, M.; Berna, J. Stereocontrolled Synthesis of β-Lactams within [2]Rotaxanes: Showcasing the Chemical Consequences of the Mechanical Bond. J. Am. Chem. Soc. 2016, 138, 8726–8729. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, V.; Capron, N.; Gase, T.; Gatti, F.G.; Kajzar, F.; Leigh, D.A.; Zerbetto, F.; Zhang, S. Influencing Intramolecular Motion with an Alternating Electric Field. Nature 2000, 406, 608–611. [Google Scholar] [PubMed]
- D’Souza, D.M.; Leigh, D.A.; Mottier, L.; Mullen, K.M.; Paolucci, F.; Teat, S.J.; Zhang, S. Nitrone [2]Rotaxanes: Simultaneous Chemical Protection and Electrochemical Activation of a Functional Group. J. Am. Chem. Soc. 2010, 132, 9465–9470. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, E.; Forbes, C.C.; Noll, B.C.; Smith, B.D. Squaraine-Derived Rotaxanes: Sterically Protected Fluorescent Near-IR Dyes. J. Am. Chem. Soc. 2005, 127, 3288–3289. [Google Scholar] [CrossRef] [PubMed]
- Gassensmith, J.J.; Baumes, J.M.; Smith, B.D. Discovery and early development of squaraine rotaxanes. Chem. Commun. 2009, 42, 6329–6338. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cuezva, A.; Berna, J.; Orenes, R.-A.; Pastor, A.; Alajarin, M. Small-Molecule Recognition for Controlling Molecular Motion in Hydrogen-Bond-Assembled Rotaxanes. Angew. Chem. Int. Ed. 2014, 53, 6762–6767. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cuezva, A.; Pastor, A.; Cioncoloni, G.; Orenes, R.-A.; Alajarin, M.; Symes, M.D.; Berna, J. Versatile control of the submolecular motion of di(acylamino)pyridine-based [2]rotaxanes. Chem. Sci. 2015, 6, 3087–3094. [Google Scholar] [CrossRef]
- Martinez-Cuezva, A.; Carro-Guillen, F.; Pastor, A.; Marin-Luna, M.; Orenes, R.-A.; Alajarin, M.; Berna, J. Co-conformational Exchange Triggered by Molecular Recognition in a Di(acylamino)pyridine-Based Molecular Shuttle Containing Two Pyridine Rings at the Macrocycle. Chem. Phys. Chem. 2016, 17, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Tron, A.; Pianet, I.; Martinez-Cuezva, A.; Tucker, J.H.R.; Pisciottani, L.; Alajarin, M.; Berna, J.; McClenaghan, N.D. Remote Photoregulated Ring Gliding in a [2]Rotaxane via a Molecular Effector. Org. Lett. 2017, 19, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Langton, M.J.; Robinson, S.W.; Marques, I.; Félix, V.; Beer, P.D. Halogen bonding in water results in enhanced anion recognition in acyclic and rotaxane hosts. Nat. Chem. 2014, 6, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Spence, G.T.; Beer, P.D. Expanding the Scope of the Anion Templated Synthesis of Interlocked Structures. Acc. Chem. Res. 2013, 46, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Berna, J.; Alajarin, M.; Orenes, R.-A. Azodicarboxamides as Template Binding Motifs for the Building of Hydrogen-Bonded Molecular Shuttles. J. Am. Chem. Soc. 2010, 132, 10741–10747. [Google Scholar] [CrossRef] [PubMed]
- Berna, J.; Alajarin, M.; Marin-Rodriguez, C.; Franco-Pujante, C. Redox divergent conversion of a [2]rotaxane into two distinct degenerate partners with different shuttling dynamics. Chem. Sci. 2012, 3, 2314–2320. [Google Scholar] [CrossRef]
- Berna, J.; Franco-Pujante, C.; Alajarin, M. Competitive binding for triggering a fluorescence response in a hydrazodicarboxamide-based [2]rotaxane. Org. Biomol. Chem. 2014, 12, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Berna, J.; Alajarin, M.; Martinez-Espin, J.S.; Buriol, L.; Martins, M.A.P.; Orenes, R.-A. Dampened circumrotation by CH⋯π interactions in hydrogen bonded [2]rotaxanes. Chem. Commun. 2012, 48, 5677–5679. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Kim, H.S.; Chang, K.-J.; Jeong, K.-S. Efficient Modulation of Hydrogen-Bonding Interactions by Remote Substituents. Org. Lett. 2004, 6, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Farràs, P.; Escudero-Adán, E.C.; Viñas, C.; Teixidor, F. Controlling the Pirouetting Motion in Rotaxanes by Counterion Exchange. Inorg. Chem. 2014, 53, 8654–8661. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.H.; Hormann, R.E. Theoretical Study of the Structure and Rotational Flexibility of Diacylhydrazines: Implications for the Structure of Nonsteroidal Ecdysone Agonists and Azapeptides. J. Am. Chem. Soc. 1996, 118, 9395–9401. [Google Scholar] [CrossRef]
- Leigh, D.A.; Murphy, A.; Smart, J.P.; Slawin, A.M.Z. Glycylglycine Rotaxanes—The Hydrogen Bond Directed Assembly of Synthetic Peptide Rotaxanes. Angew. Chem. Int. Ed. Engl. 1997, 36, 728–732. [Google Scholar] [CrossRef]
- Gatti, F.G.; Leigh, D.A.; Nepogodiev, S.A.; Slawin, A.M.Z.; Teat, S.J.; Wong, J.K.Y. Stiff, and sticky in the right places: The dramatic influence of preorganizing guest binding sites on the hydrogen bond-directed assembly of rotaxanes. J. Am. Chem. Soc. 2001, 123, 5983–5989. [Google Scholar] [CrossRef] [PubMed]
- Gatti, F.G.; León, S.; Wong, J.K.Y.; Bottari, G.; Altieri, A.; Morales, M.A.F.; Teat, S.J.; Frochot, C.; Leigh, D.A.; Brouwer, A.M.; et al. Photoisomerization of a rotaxane hydrogen bonding template: Light-induced acceleration of a large amplitude rotational motion. Proc. Natl. Acad. Sci. USA 2003, 100, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Altieri, A.; Gatti, F.G.; Kay, E.R.; Leigh, D.A.; Martel, D.; Paolucci, F.; Slawin, A.M.Z.; Wong, J.K.Y. Electrochemically switchable hydrogen-bonded molecular shuttles. J. Am. Chem. Soc. 2003, 125, 8644–8654. [Google Scholar] [CrossRef] [PubMed]
- Murgu, I.; Baumes, J.M.; Eberhard, J.; Gassensmith, J.J.; Arunkumar, E.; Smith, B.D. Macrocycle breathing in [2]rotaxanes with tetralactam macrocycles. J. Org. Chem. 2011, 76, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Umezawa, Y.; Fantini, J.; Weiss, M.S.; Chakrabarti, P. CH-π hydrogen bonds in biological macromolecules. Phys. Chem. Chem. Phys. 2014, 16, 12648–12683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, Y.; Chen, J.; Dong, S.; Yu, Y.; Ma, Z.; Huang, F. Formation of Linear Supramolecular Polymers That Is Driven by CH⋅⋅⋅π Interactions in Solution and in the Solid State. Angew. Chem. Int. Ed. 2011, 50, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Entry | Rotaxane | δ (HEeq); δ (HE’ax) ppm | Δν/Hz | kc/s−1 | Tc 1/K | ΔG≠ 2/kcal mol−1 |
|---|---|---|---|---|---|---|
| 1 | [2H]-3 | 4.64; 4.33 | 128 | 290 | 228 | 10.7 |
| 2 | 3 | 4.96; 4.06 | 360 | 802 | 413 | 19.1 |
| 3 | 4 | 4.98; 4.00 | 392 | 873 | 408 | 18.6 |
| 4 | 5 | 4.96; 4.07 | 356 | 793 | 413 | 19.0 |
| 5 | [2H]-7 3 | 4.78; 4.22 | 237 | 527 | 228 | 10.4 |
| 6 | 73 | 5.14; 3.67 | 560 | 1245 | 333 | 14.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saura-Sanmartin, A.; Martinez-Espin, J.S.; Martinez-Cuezva, A.; Alajarin, M.; Berna, J. Effects on Rotational Dynamics of Azo and Hydrazodicarboxamide-Based Rotaxanes. Molecules 2017, 22, 1078. https://doi.org/10.3390/molecules22071078
Saura-Sanmartin A, Martinez-Espin JS, Martinez-Cuezva A, Alajarin M, Berna J. Effects on Rotational Dynamics of Azo and Hydrazodicarboxamide-Based Rotaxanes. Molecules. 2017; 22(7):1078. https://doi.org/10.3390/molecules22071078
Chicago/Turabian StyleSaura-Sanmartin, Adrian, Juan S. Martinez-Espin, Alberto Martinez-Cuezva, Mateo Alajarin, and Jose Berna. 2017. "Effects on Rotational Dynamics of Azo and Hydrazodicarboxamide-Based Rotaxanes" Molecules 22, no. 7: 1078. https://doi.org/10.3390/molecules22071078