Characterization of Polyelectrolyte Complex Formation Between Anionic and Cationic Poly(amino acids) and Their Potential Applications in pH-Dependent Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Poly(l-glutamic acid)/Poly(l-lysine) PECs
2.2. Recombinant Expression of (HE)20 Peptide
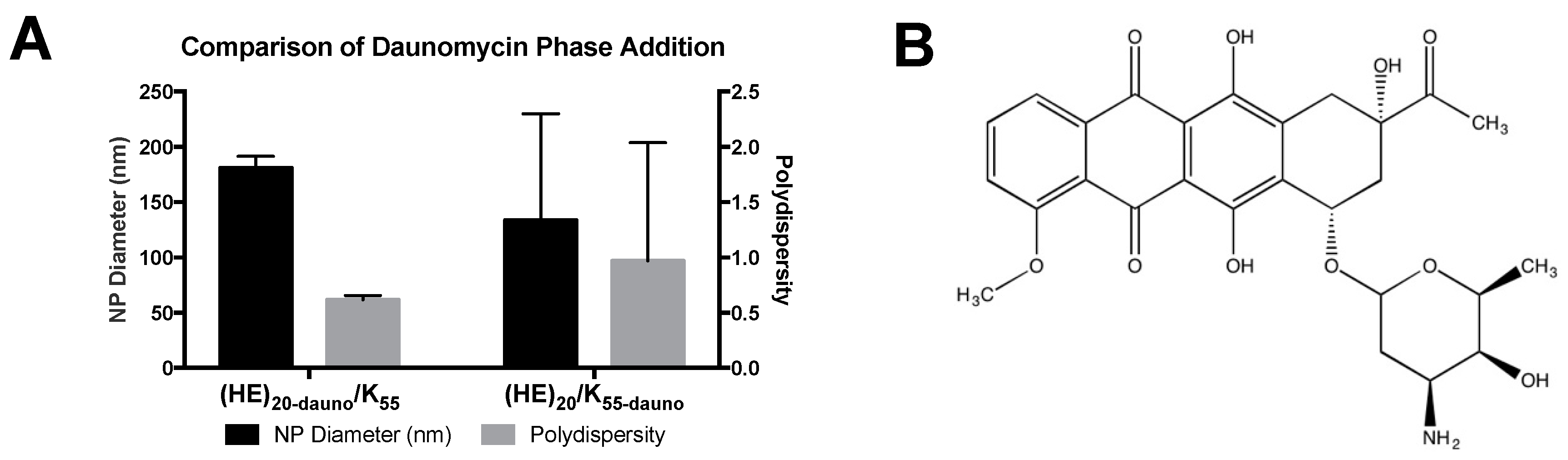
2.3. Formulation of PEC with Daunomycin, (HE)20, and PLys55
2.4. Formulation of PEC with Daunomycin, (HE)20-dauno, and K55 in ZnCl2·2NH4Cl solution
2.5. Formulation of PEC with Daunomycin, E51, and K55 in ZnCl2·2NH4Cl solution
2.6. pH Sensitivity of E51-dauno/K55-Zn PEC and (HE)20-dauno/K55-Zn
3. Results and Discussion
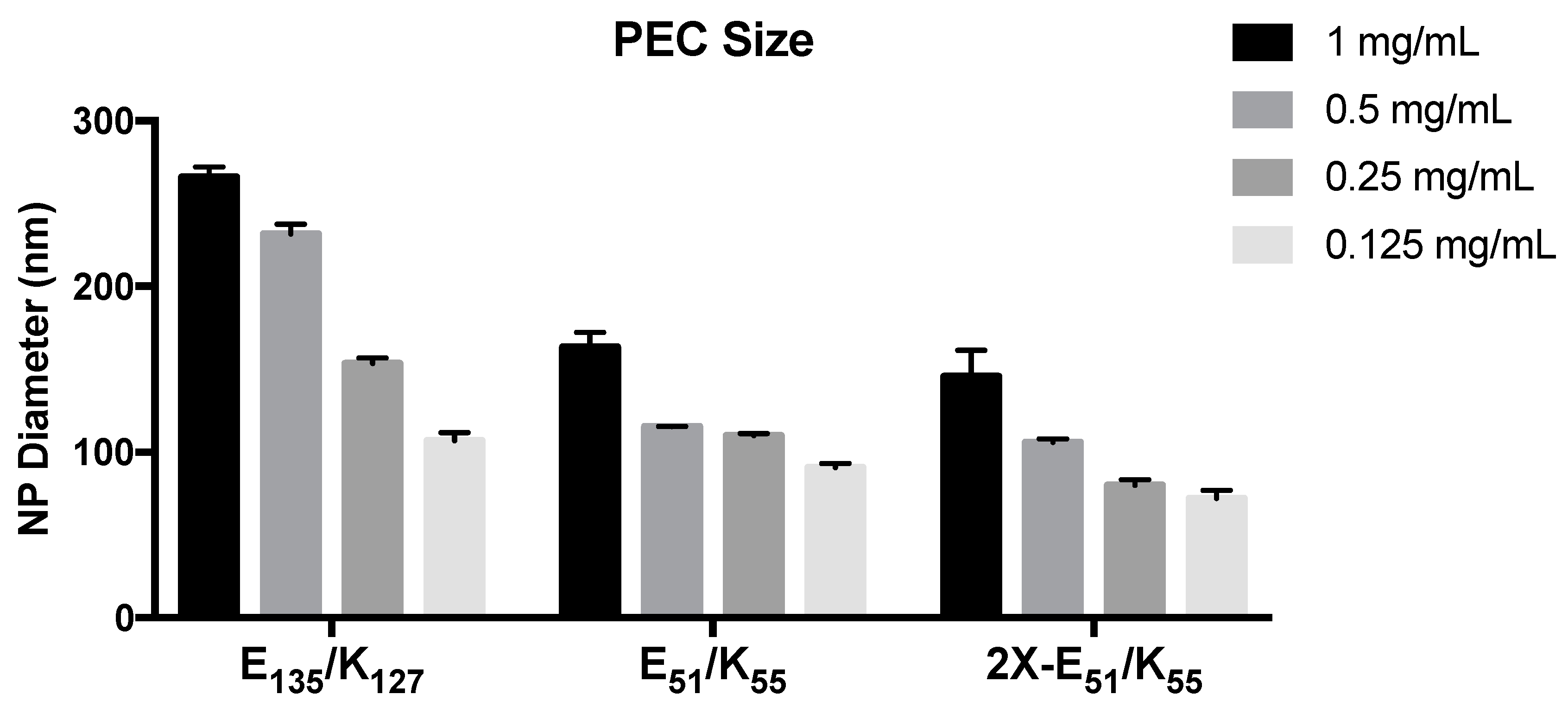
3.1. PEC Formed from Kn and En
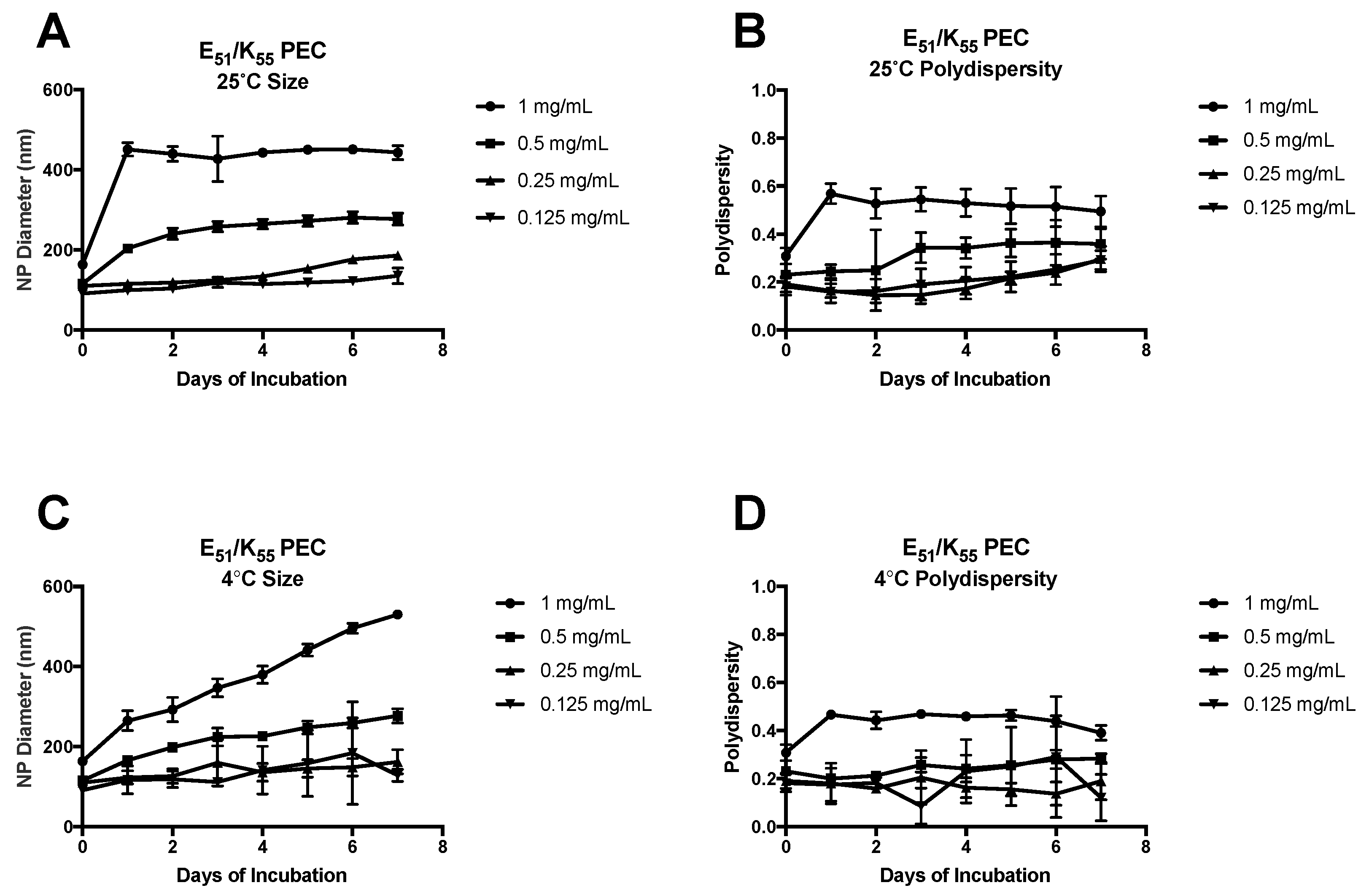
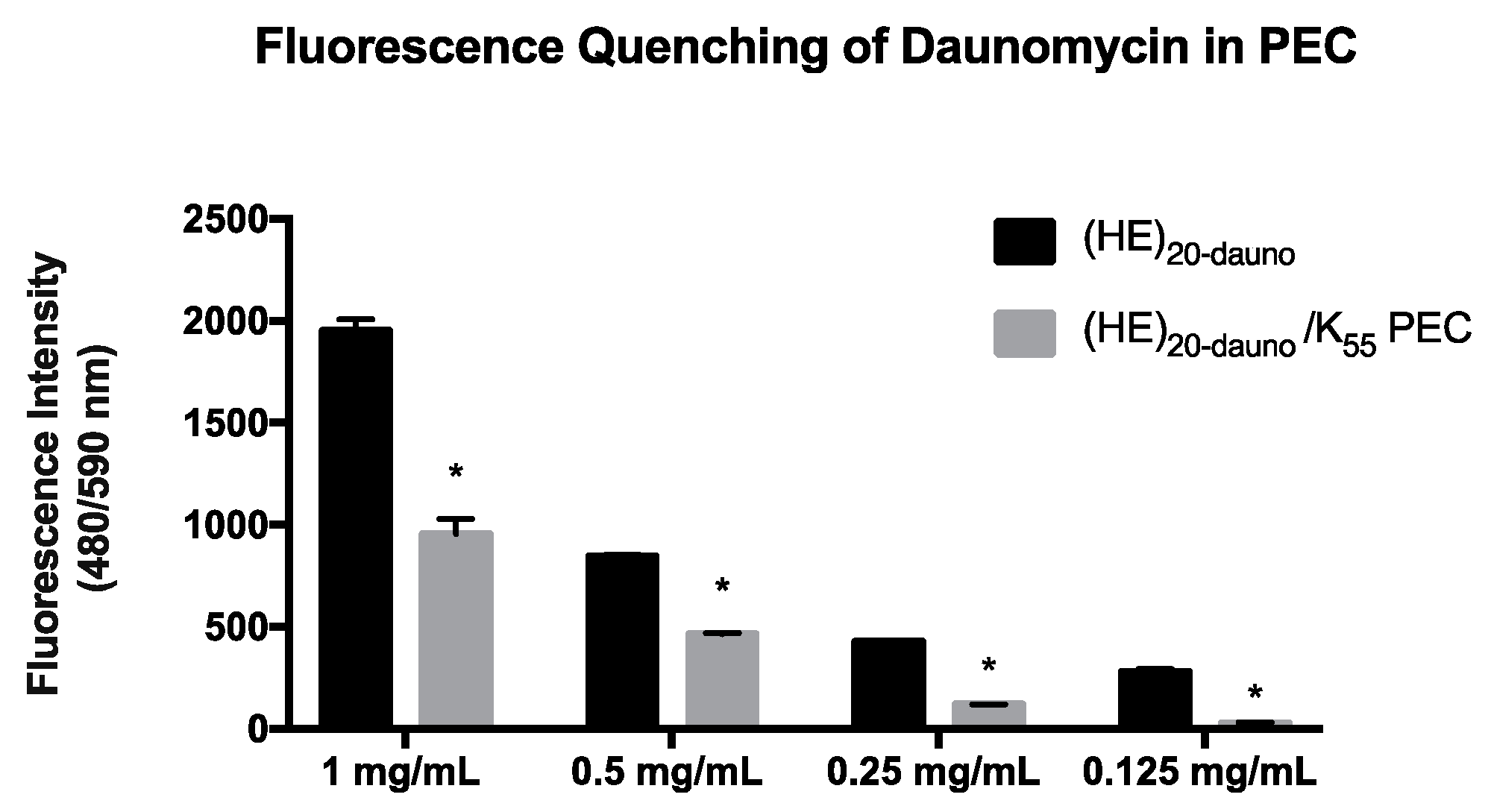
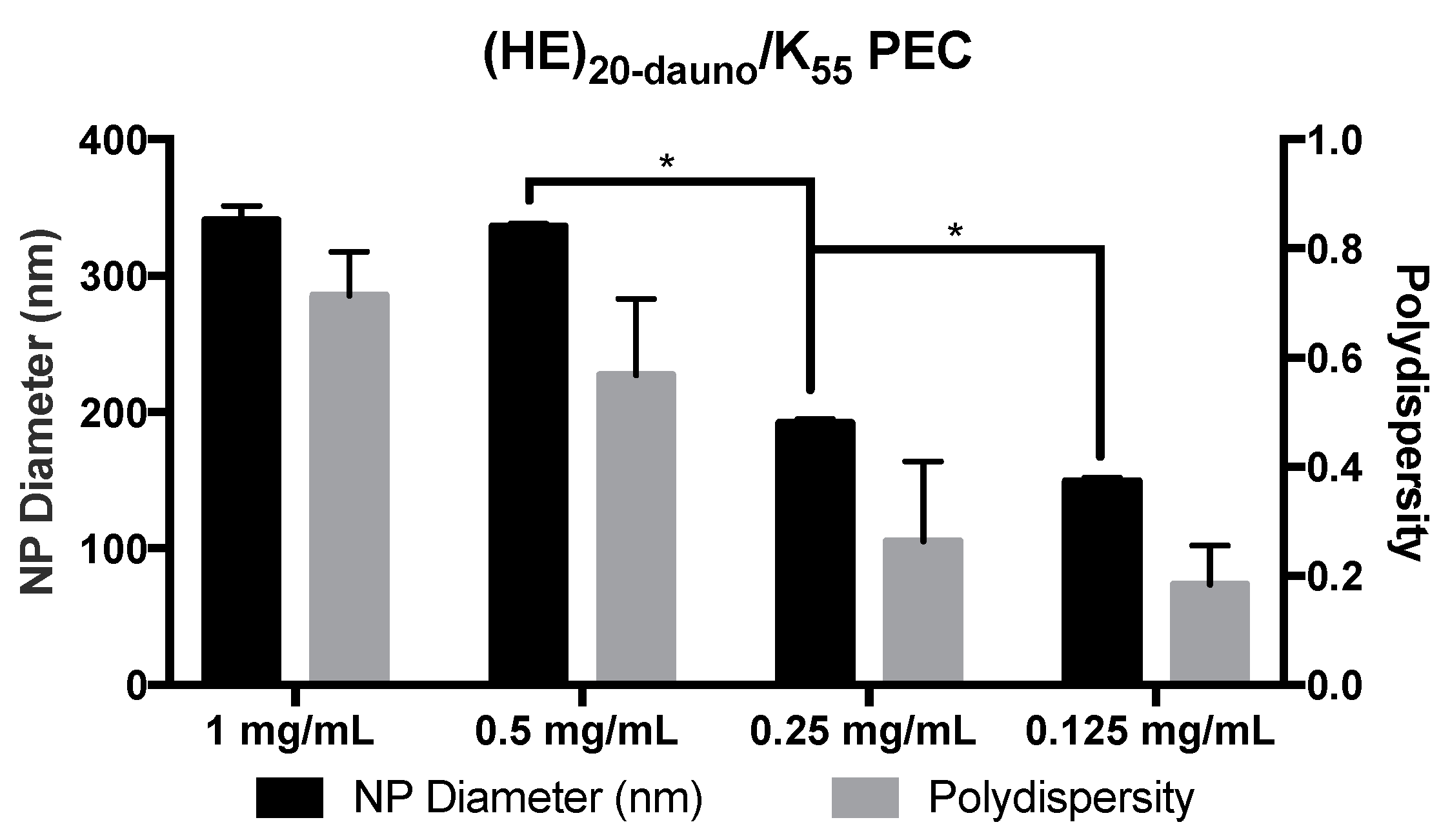
3.2. Formulation of PEC with Daunomycin, (HE)20, and K55
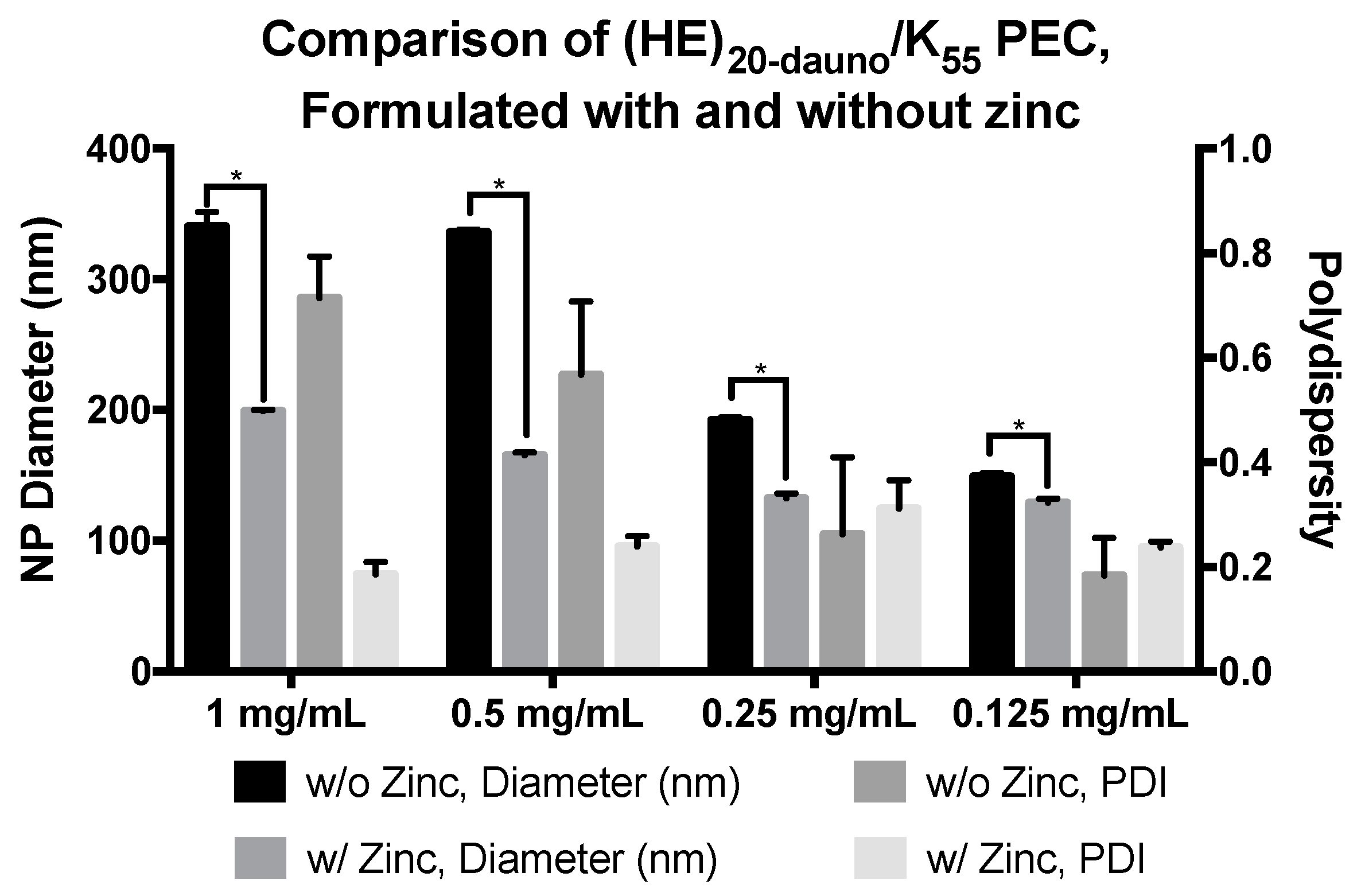
3.3. Formulation of PEC with Daunomycin, (HE)20, and K55 in ZnCl2·2NH4Cl Solution
3.4. pH Sensitivity of E51-dauno/K55-Zn PEC and (HE)20-dauno/K55-Zn
4. Summary
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartig, S.M.; Greene, R.R.; Dikov, M.M.; Prokop, A.; Davidson, J.M. Multifunctional nanoparticulate polyelectrolyte complexes. Pharm. Res. 2007, 24, 2353–2369. [Google Scholar] [CrossRef] [PubMed]
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147–148, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Domard, A.; Rinaudo, M. Polyelectrolyte Complexes. Interaction of Poly(l-lysine)-Poly(l-glutamic acid) in Dilute Aqueous Solution. Macromolecules 1980, 13, 898–904. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte complexes: Mechanisms, critical experimental aspects, and applications. Artif. Cells Nanomed. Biotechnol. 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ma, C.; Li, C.; Wang, T.; Tang, Y.; Wang, H.; Moul, X.; Chen, Z.; Hel, N. Influence of nanoparticle shape, size, and surface functionalization on cellular uptake. J. Nanosci. Nanotechnol. 2013, 13, 6485–6498. [Google Scholar] [CrossRef] [PubMed]
- Rasente, R.Y.; Imperiale, J.C.; Lazaro-Martinez, J.M.; Gualco, L.; Oberkersch, R.; Sosnik, A.; Calabrese, G.C. Dermatan sulfate/chitosan polyelectrolyte complex with potential application in the treatment and diagnosis of vascular disease. Carbohydr. Polym. 2016, 144, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.G.; Dong, L.C. Characterization of physiochemical and biological properties of an insulin/lauryl sulfate complex formed by hydrophobic ion pairing. Int. J. Pharm. 2007, 336, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cui, F.; Shi, K.; Cun, D.; Wang, R. Design of high payload PLGA nanoparticles containing melittin/sodium dodecyl sulfate complex by the hydrophobic ion-pairing technique. Drug Dev. Ind. Pharm. 2009, 35, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liang, N.; Kawashima, Y.; Xia, D.; Cui, F. Hydrophobic ion pairing of an insulin-sodium deoxycholate complex for oral delivery of insulin. Int. J. Nanomed. 2011, 6, 3049–3056. [Google Scholar] [CrossRef]
- Muller, M.; Reihs, T.; Ouyang, W. Needlelike and spherical polyelectrolyte complex nanoparticles of poly(l-lysine) and copolymers of maleic acid. Langmuir ACS J. Surf. Colloids 2005, 21, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Reihs, T.; Muller, M.; Lunkwitz, K. Preparation and adsorption of refined polyelectrolyte complex nanoparticles. J. Colloid Interface Sci. 2004, 271, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Kaneko, T.; Kida, T.; Akashi, M. Preparation and characterization of biodegradable nanoparticles based on poly(gamma-glutamic acid) with l-phenylalanine as a protein carrier. J. Control. Release Off. J. Control. Release Soc. 2005, 108, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Watanabe, K.; Kim, H.; Akashi, M. Stabilization of polyion complex nanoparticles composed of poly(amino acid) using hydrophobic interactions. Langmuir 2010, 26, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.X.; Lin, D.Q.; Yao, S.J. Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Mar. Drugs 2014, 12, 6236–6253. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Parolin, C.; Vitali, B.; Bigucci, F.; Gallucci, M.C.; Nicoletta, F.P.; Luppi, B. Microparticles based on chitosan/carboxymethylcellulose polyelectrolyte complexes for colon delivery of vancomycin. Carbohydr. Polym. 2016, 143, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Mocchiutti, P.; Schnell, C.N.; Rossi, G.D.; Peresin, M.S.; Zanuttini, M.A.; Galvan, M.V. Cationic and anionic polyelectrolyte complexes of xylan and chitosan. Interaction with lignocellulosic surfaces. Carbohydr. Polym. 2016, 150, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H. Chitosan Based Polyelectrolyte Complexes as Potential Carrier Materials in Drug Delivery Systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Delair, T. Colloidal polyelectrolyte complexes of chitosan and dextran sulfate towards versatile nanocarriers of bioactive molecules. Eur. J. Pharm. Biopharm 2011, 78, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.C. Acid-Sensitive Dissociation between Poly(Lysine) and Histamine-Modified Poly(Glutamate) as a Model for Drug-Releasing from Carriers in Endosomes. Biochim. Biophys. Acta 1990, 1034, 122–124. [Google Scholar] [CrossRef]
- Zaro, J.L.; Fei, L.; Shen, W.C. Recombinant peptide constructs for targeted cell penetrating peptide-mediated delivery. J. Control. Release 2012, 158, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-H.; Chen, Y.-R.; Chen, S.-Y.; Shen, W.-C.; Ann, D.K.; Zaro, J.L.; Shen, L.-J. Selective intracellular delivery of recombinant arginine deiminase (ADI) using pH-sensitive cell penetrating peptides to overcome ADI resistance in hypoxic breast cancer cells. Mol. Pharm. 2015, 13, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Yap, L.P.; Conti, P.S.; Shen, W.C.; Zaro, J.L. Tumor targeting of a cell penetrating peptide by fusing with a pH-sensitive histidine-glutamate co-oligopeptide. Biomaterials 2014, 35, 4082–4087. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.M.; Shen, W.C.; Tu, J.S.; Zaro, J.L. Interaction between Cell-Penetrating Peptides and Acid-Sensitive Anionic Oligopeptides as a Model for the Design of Targeted Drug Carriers. Mol. pharm. 2014, 11, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Bains, O.S.; Szeitz, A.; Lubieniecka, J.M.; Cragg, G.E.; Grigliatti, T.A.; Riggs, K.W.; Reid, R.E. A Correlation between Cytotoxicity and Reductase-Mediated Metabolism in Cell Lines Treated with Doxorubicin and Daunorubicin. J. Pharm. Exp. Ther. 2013, 347, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Htun, M.T. Photophysical study on daunorubicin by fluuorescence spectroscopy. J. Lumin. 2009, 129, 344–348. [Google Scholar] [CrossRef]
- Starchenko, V.; Muller, M.; Lebovka, N. Sizing of PDADMAC/PSS complex aggregates by polyelectrolyte and salt concentration and PSS molecular weight. J. Phys. Chem. B 2012, 116, 14961–14967. [Google Scholar] [CrossRef] [PubMed]
- Buchhammer, H.M.; Mende, M.; Oelmann, M. Formation of mono-sized polyelectrolyte complex dispersions: Effects of polymer structure, concentration and mixing conditions. Colloid Surf. A 2003, 218, 151–159. [Google Scholar] [CrossRef]
- Wandrey, C.; Hunkeler, D.; Wendler, U.; Jaeger, W. Counterion activity of highly charged strong polyelectrolytes. Macromolecules 2000, 33, 7136–7143. [Google Scholar] [CrossRef]
- Muller, M.; Kessler, B.; Frohlich, J.; Poeschla, S.; Torger, B. Polyelectrolyte Complex Nanoparticles of Poly(ethyleneimine) and Poly(acrylic acid): Preparation and Applications. Polymers 2011, 3, 762–778. [Google Scholar] [CrossRef]
- Gallois, L.; Fiallo, M.; Garnier-Suillerot, A. Comparison of the interaction of doxorubicin, daunorubicin, idarubicin and idarubicinol with large unilamellar vesicles—Circular dichroism study. Bba-Biomembranes 1998, 1370, 31–40. [Google Scholar] [CrossRef]
- Sangster, J. Octanol-Water Partition-Coefficients of Simple Organic-Compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111–1229. [Google Scholar] [CrossRef]
- Kolitz-Domb, M.; Corem-Salkmon, E.; Grinberg, I.; Margel, S. Synthesis and characterization of bioactive conjugated near-infrared fluorescent proteinoid-poly(l-lactic acid) hollow nanoparticles for optical detection of colon cancer. Int. J. Nanomed. 2014, 9, 5041–5053. [Google Scholar] [CrossRef]
- Joshi, S.; Husain, M.M.; Chandra, R.; Hasan, S.K.; Srivastava, R.C. Hydroxyl radical formation resulting from the interaction of nickel complexes of l-histidine, glutathione or l-cysteine and hydrogen peroxide. Hum. Exp. Toxicol. 2005, 24, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.M.; Sunde, M. Zinc fingers–Folds for many occasions. IUBMB Life 2002, 54, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.L.; Besold, A.N.; Michel, S.L. Cysteine and histidine shuffling: Mixing and matching cysteine and histidine residues in zinc finger proteins to afford different folds and function. Dalton Trans. 2011, 40, 12619–12632. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517. [Google Scholar] [CrossRef]
- Higuchi, T.; Kotwal, P.M. Complexes of Doxorubicin Exhibiting Enhanced Stability. U.S. Patents 4,246,399 A, 20 January 1981. [Google Scholar]
- Barick, K.; Nigam, S.; Bahadur, D. Nanoscale assembly of mesoporous ZnO: A potential drug carrier. J. Mater. Chem. 2010, 20, 6446–6452. [Google Scholar] [CrossRef]
- Ravindran, S.; Ozkan, C.S. Self-assembly of ZnO nanoparticles to electrostatic coordination sites of functionalized carbon nanotubes. Nanotechnology 2005, 16, 1130. [Google Scholar] [CrossRef]
- Jochum, T.; Reddy, C.M.; Eichhöfer, A.; Buth, G.; Szmytkowski, J.; Kalt, H.; Moss, D.; Balaban, T.S. The supramolecular organization of self-assembling chlorosomal bacteriochlorophyll c, d, or e mimics. Proc. Natl. Acad. Sci. USA 2008, 105, 12736–12741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, J.J.; Zhang, B.Z.; Liu, L.W.; Wang, K.R.; Wang, R. Design of Acid-Activated Cell Penetrating Peptide for Delivery of Active Molecules into Cancer Cells. Bioconj. Chem. 2011, 22, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Huang, Y.W.; Winiarz, J.G.; Chiang, H.J.; Lee, H.J. Intracellular delivery of quantum dots mediated by a histidine- and arginine-rich HR9 cell-penetrating peptide through the direct membrane translocation mechanism. Biomaterials 2011, 32, 3520–3537. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.P.; Uthaman, S.; John, J.V.; Lee, H.R.; Lee, S.J.; Park, H.; Park, I.K.; Suh, H.; Kim, I. Poly(PEGA)-b-poly(l-lysine)-b-poly(l-histidine) Hybrid Vesicles for Tumoral pH-Triggered Intracellular Delivery of Doxorubicin Hydrochloride. ACS Appl. Mater. Interfaces 2015, 7, 21770–21779. [Google Scholar] [CrossRef] [PubMed]
- Bagherifam, S.; Skjeldal, F.M.; Griffiths, G.; Maelandsmo, G.M.; Engebraten, O.; Nystrom, B.; Hasirci, V.; Hasirci, N. pH-Responsive nano carriers for doxorubicin delivery. Pharm. Res. 2015, 32, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhu, L.; Torchilin, V.P. pH-Sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Liu, C.G.; Wang, X.L.; Huang, Z.H. Preparation and characterization of nanoparticles based on histidine-hyaluronic acid conjugates as doxorubicin carriers. J Mater. Sci. Mater. Med. 2012, 23, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Upreti, M.; Jyoti, A.; Sethi, P. Tumor microenvironment and nanotherapeutics. Transl. Cancer Res. 2013, 2, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Zhou, K.J.; Huang, G.; Hensley, C.; Huang, X.N.; Ma, X.P.; Zhao, T.; Sumer, B.D.; DeBerardinis, R.J.; Gao, J.M. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 2014, 13, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Omidi, Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts 2013, 3, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging JMRI 2002, 16, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bhujwalla, Z.M.; Artemov, D.; Ballesteros, P.; Cerdan, S.; Gillies, R.J.; Solaiyappan, M. Combined vascular and extracellular pH imaging of solid tumors. NMR Biomed. 2002, 15, 114–119. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Daunomycin Concentration (mg/mL) | Encapsulation Efficiency, % | Loading Content, wt % |
|---|---|---|
| 1 | 68% | 25% |
| 0.5 | 58% | 21% |
| 0.25 | 74% | 27% |
| 0.125 | 85% | 31% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folchman-Wagner, Z.; Zaro, J.; Shen, W.-C. Characterization of Polyelectrolyte Complex Formation Between Anionic and Cationic Poly(amino acids) and Their Potential Applications in pH-Dependent Drug Delivery. Molecules 2017, 22, 1089. https://doi.org/10.3390/molecules22071089
Folchman-Wagner Z, Zaro J, Shen W-C. Characterization of Polyelectrolyte Complex Formation Between Anionic and Cationic Poly(amino acids) and Their Potential Applications in pH-Dependent Drug Delivery. Molecules. 2017; 22(7):1089. https://doi.org/10.3390/molecules22071089
Chicago/Turabian StyleFolchman-Wagner, Zoë, Jennica Zaro, and Wei-Chiang Shen. 2017. "Characterization of Polyelectrolyte Complex Formation Between Anionic and Cationic Poly(amino acids) and Their Potential Applications in pH-Dependent Drug Delivery" Molecules 22, no. 7: 1089. https://doi.org/10.3390/molecules22071089





