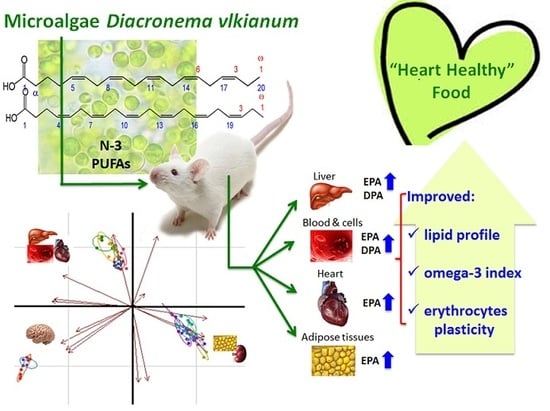
Evaluation of Marine Microalga Diacronema vlkianum Biomass Fatty Acid Assimilation in Wistar Rats
Abstract
:1. Introduction
2. Results
2.1. Diet Fatty Acid Profile
2.2. Animal Dosing Studies
2.3. Biochemical Parameters
2.4. Cells and Tissue Fatty Acid Composition
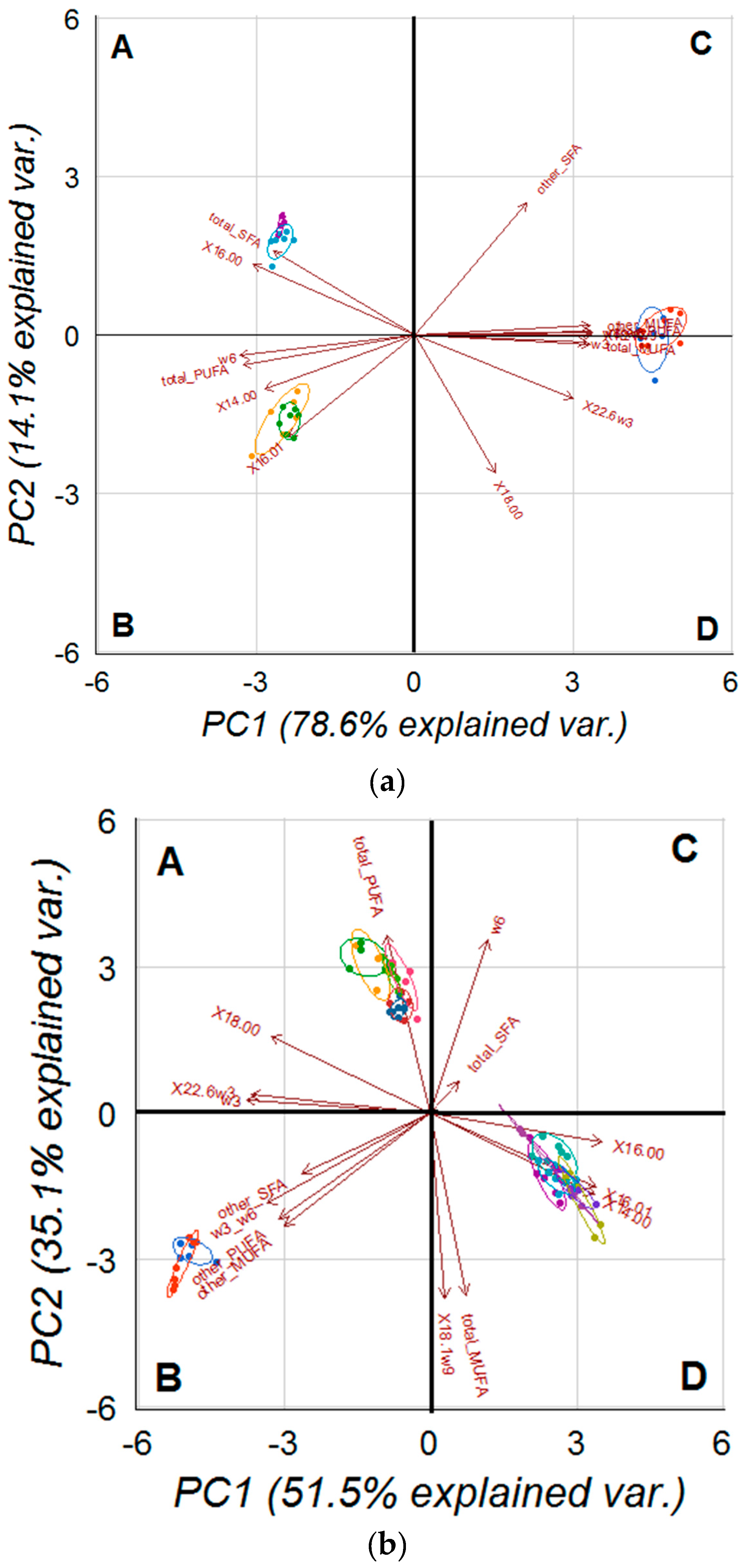
2.5. PCA on Analysed Cell and Tissues Fatty Acids Profile
3. Discussion
4. Materials and Methods
4.1. Microalgae Biomass Production
4.2. Animals
4.3. Animal Studies
4.3.1. Single Dose Mice Study
4.3.2. Repeated Dose Rat Study
4.4. Analysis of Erythrocytes and Plasma Biochemical Parameters
4.5. Analysis of Fatty Acid Composition
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schrijver, R.D.; Vermeulen, D.; Backx, S. Digestion and Absorption of Free and Esterified Fish Oil Fatty Acids in Rats. Lipids 1991, 26, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Sousa, J.; Moura, P.; Bandarra, N.M. Microalgae-source of natural bioactives as functional ingredients. Food Sci. Technol. Bull. 2010, 7, 21–37. [Google Scholar] [CrossRef]
- Behrens, P.W.; Kyle, D.J. Microalgae as a source of fatty acids. J. Food Lipid 1996, 3, 259–272. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Fatty acids and cardiovascular disease: Evidence explained and mechanisms explored. Clin. Sci. 2004, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Isochrysis galbana and Diacronema vlkianum biomass incorporation in pasta products as PUFA’s source. LWT Food Sci. Technol. 2013, 50, 312–319. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Cannon, D. From fish oil to microalgae oil: A win-win shift for humans and our habitat. Explore 2009, 5, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Beelen, V.A.; Spenkelink, B.; Mooibroek, H.; Sijtsma, L.; Bosch, D.; Rietjens, I.M.C.M.; Alink, G.M. An n-3 PUFA-rich microalga oil diet protects to a similar extent as a fish oil-rich diet against AOM-induced colonic aberrant crypt foci in F344 rats. Food Chem. Toxicol. 2009, 47, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Durmaz, Y.; Donato, M.; Monteiro, M.; Gouveia, L.; Nunes, M.L.; Gama Pereira, T.; Gokpınar, S.; Bandarra, N.M. Effect of temperature on a-tocopherol, fatty acid profile, and pigments of Diacronema vlkianum (Haptophyceae). Aquacult. Int. 2009, 17, 391–399. [Google Scholar] [CrossRef]
- Donato, M.; Vilela, H.M.; Bandarra, N.M. Fatty acids, sterols, α-tocopherol and total carotenoids composition of Diacronema vlkianum. J. Food Lipid 2003, 10, 267–276. [Google Scholar] [CrossRef]
- Volkman, J.K.; Farmer, C.L.; Barrett, S.M.; Sikes, E.L. Unusual dihydroxysterols as chemotaxonomic markers for microalgae from the order Pavlovales (Haptophyceae). J. Phycol. 1997, 33, 1016–1023. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER). Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Food and Drug Adminstration: Rockville, MD, USA, 2005.
- Buse, J.B.; Ginsberg, H.N.; Bakris, G.L.; Clark, N.G.; Costa, F.; Eckel, R.; Fonseca, V.; Gerstein, H.C.; Grundy, S.; Nesto, R.W.; et al. Primary Prevention of Cardiovascular Diseases in People With Diabetes Mellitus A Scientific Statement from the American Heart Association and the American Diabetes Association. Circulation 2007, 115, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.; Atar, D.; Borch-Johnsen, K.; Boysen, G.; Burell, G.; Cifkova, R.; Dallongeville, J.; De Backer, G.; Ebrahim, S.; Gjelsvik, B.; et al. European guidelines on cardiovascular disease prevention in clinical practice: Executive summary. Atherosclerosis 2007, 194, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C., Jr.; Allen, J.; Blair, S.N.; Bonow, R.O.; Brass, L.M.; Fonarow, G.C.; Grundy, S.M.; Hiratzka, L.; Jones, D.; Krumholz, H.M.; et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006, 113, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ohta, M.; Nakakuki, M.; Kami, H.; Uchiyama, R.; Kawano, H.; Notsu, T.; Imada, K.; Shimano, H. Distinct regulation of plasma LDL cholesterol by eicosapentaenoic and docosahexaenoic acid in high fat diet- fed hamsters: Participation of cholesterol ester transfer protein and LDL receptor. Prostaglandins Leukot Essent Fatty Acids 2013, 88, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, R.C.S.; de Mello-Sampayo, C.; Antoniazzi, C.T.D.; Segat, H.J.; Silva, H.; Veit, J.C.; Piccolo, T.E.; Bürger, M.E.; Silva-Lima, B.; Rodrigues, L.M. Oral supplementation with fish oil reduces dryness and pruritus in the acetone-induced dry skin rat model. J. Dermatol. Sci. 2015, 79, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Lopes, P.A.; Martins, S.V.; Ferreira, J.; Alfaia, C.M.; Rolo, E.A.; Correia, J.J.; Pinto, R.M.; Ramos-Bueno, R.P.; Batista, I.; et al. Docosahexaenoic acid at the sn-2 position of structured triacylglycerols improved n-3 polyunsaturated fatty acid assimilation in tissues of hamsters. Nutr. Res. 2016, 36, 452–463. [Google Scholar] [CrossRef]
- Lee, K.T.; Akoh, C.C.; Dawe, D.L. Effects of structured lipid containing omega 3 and medium chain fatty acids on serum lipids and immunological variables in mice. J. Food Biochem. 1999, 23, 197–208. [Google Scholar] [CrossRef]
- Mori, T.A.; Watts, G.F.; Burke, V.; Hilme, E.; Puddey, I.B.; Beilin, L.J. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation 2000, 102, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 Fatty Acids, Hepatic Lipid Metabolism, and Nonalcoholic Fatty Liver Disease. Annu. Rev. Nutr. 2013, 33, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Angeli, M.T.; Colli, S.; Eligini, S.; Tremoli, E.; Sirtori, C.R.; Galli, C. Changes of n-3 and n-6 fatty acids in plasma and circulating cells of normal subjects, after prolonged administration of 20:5 (EPA) and 22:6 (DHA) ethyl esters and prolonged washout. Biochim. Biophys. Acta 1993, 1210, 55–62. [Google Scholar] [CrossRef]
- De Mello-Sampayo, C.; Corvo, M.L.; Mendes, R.; Duarte, D.; Lucas, J.; Pinto, R.; Batista, A.P.; Raymundo, A.; Silva-Lima, B.; Bandarra, N.M.; Gouveia, L. Insights on the safety of carotenogenic Chlorella vulgaris in rodents. Algal Res. 2013, 2, 409–415. [Google Scholar] [CrossRef]
- Woo, S.; Jusko, W.J. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab. Dispos. 2007, 35, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.A.; Riordan, H.D.; Hunninghake, R.; Meng, X.; Sarwar, Y. Red blood cell membrane fatty acids as a diagnostic test. J. Orthomol. Med. 1997, 12, 20–22. [Google Scholar]
- Harris, W.S.; Sands, S.A.; Windsor, S.L.; Ali, H.A.; Stevens, T.L.; Magalski, A.; Porter, C.B.; Borkon, A.M. Omega-3 Fatty Acids in Cardiac Biopsies From Heart Transplantation Patients - Correlation With Erythrocytes and Response to Supplementation. Circulation 2004, 110, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [PubMed]
- Ku, C.S.; Pham, T.X.; Park, Y.; Kim, B.; Shin, M.S.; Kang, I.; Lee, J. Edible blue-green algae reduce the production of pro-inflammatory cytokines by inhibiting NF-kB pathway in macrophages and splenocytes. Biochim. Biophys. Acta 2013, 1830, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Sardi, L.; Martelli, G.; Lambertini, L.; Parisini, P.; Mordenti, A. Effects of a dietary supplement of DHA-rich marine algae on Italian heavy pig production parameters. Livest. Sci. 2006, 103, 95–103. [Google Scholar] [CrossRef]
- Sukenik, A.; Takahashi, H.; Mokady, S. Dietary lipids from marine unicellular algae enhance the amount of liver and blood omega-3 fatty acids in rats. Ann. Nutr. Metab. 1994, 38, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Torres-Durán, P.V.; Paredes-Carbajal, M.C.; Mascher, D.; Zamora-González, J.; Díaz-Zagoya, J.C.; Juárez-Oropeza, M.A. Protective effect of Arthrospira maxima on fatty acid composition in fatty liver. Arch. Med. Res. 2006, 37, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Baylin, A.; Kabagambe, E.K.; Siles, X.; Campos, H. Adipose tissue biomarkers of fatty acid intake. Am. J. Clin. Nutr. 2002, 76, 750–757. [Google Scholar] [PubMed]
- Taniguchi, H.; Suzuki, K.; Takita, T.; Chung, S.Y.; Hayakawa, T.; Nakamura, K.; Innami, S. Comparative effects of eicosapentaenoic acid and docosahexaenoic acid on n-6 and n-3 fatty acid profiles of phospholipid classes in several tissues of rats fed a hypertriglyceridemic diet. J. Clin. Biochem. Nutr. 1993, 14, 151–162. [Google Scholar] [CrossRef]
- Saito, M.; Ueno, M.; Kubo, K.; Yamaguchi, M. Dose-response effect of dietary docosahexaenoic acid on fatty acid profiles of serum and tissue lipids in rats. J. Agric. Food Chem. 1998, 46, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Green, P.; Glozman, S.; Kamensky, B.; Yavin, E. Developmental changes in rat brain membrane lipids and fatty acids—The preferential prenatal accumulation of docosahexaenoic acid. J. Lipid Res. 1999, 40, 960–966. [Google Scholar] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Covaci, A.; Voorspoels, S.; Thomsen, C.; van Bavel, B.; Neels, H. Evaluation of total lipids using enzymatic methods for the normalization of persistent organic pollutant levels in serum. Sci. Total Environ. 2006, 366, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M.; Christie, W.W. Seasonal changes in lipid composition of sardine Sardinapilchardus. J. Food Sci. 1997, 62, 40–43. [Google Scholar] [CrossRef]
- Kadegowda, A.K.; Pipierova, L.S.; Erdman, R.A. Principal component and multivariate analysis of milk long-chain fatty acid composition during diet-induced milk fat depression. J. Dairy Sci. 2007, 91, 749–759. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2014. Available online: http://www.R-project.org/ (accessed on 1 February 2017).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; p. 213. [Google Scholar]
Sample Availability: Samples of the microalgae are available. |
| Fatty Acids | Regular Diet | Regular Diet + Microalga | ||
|---|---|---|---|---|
| mg/kg | (%) | mg/kg | (%) | |
| 14:0 | 8.9 ± 0.4 | 0.5 | 61.0± 1.0 | 3.2 |
| 16:0 | 323.7 ± 16.2 | 19.3 | 359.0± 11.2 | 17.6 |
| 18:0 | 32.1 ± 1.6 | 1.9 | 32.4± 1.1 | 1.6 |
| Other SFA | 15.7 ± 0.8 | 0.9 | 17.6 ± 0.7 | 0.9 |
| Σ SFA | 380.3 ± 19.0 | 22.7 | 470.0 ± 14.0 | 23.2 |
| 16:1 | 7.5 ± 0.4 | 0.4 | 68.1 ± 1.0 | 3.6 |
| 18:1 | 305.8 ± 15.3 | 18.2 | 312.1 ± 10.3 | 15.2 |
| Other MUFA | 37.3 ± 1.9 | 2.2 | 42.1 ± 1.6 | 2.1 |
| Σ MUFA | 350.5 ± 17.5 | 20.9 | 422.3 ± 12.8 | 20.8 |
| 18:2n-6 (LA) | 835.3 ± 41.8 | 49.8 | 836.5 ± 28.9 | 40.6 |
| 20:4n-6 (AA) | 2.2 ± 0.1 | 0.1 | 7.0 ± 0.1 | 0.4 |
| Other n-6 | 82.0 ± 2.8 | 4.9 | 109.6 ± 1.1 | 5.5 |
| 18:4n-3 | 0.0 ± 0.0 | 0.0 | 28.0 ± 0.2 | 1.5 |
| 20:5n-3 (EPA) | 7.5 ± 0.4 | 0.4 | 87.8 ± 0.8 | 4.7 |
| 22:5n-3 (DPA) | 0.0 ± 0.0 | 0.0 | 16.3 ± 0.5 | 0.9 |
| 22:6n-3 (DHA) | 7.5 ± 0.4 | 0.4 | 28.4 ± 0.4 | 1.5 |
| Other n-3 | 14.9 ± 2.0 | 0.9 | 20.9 ± 2.5 | 1.0 |
| Σ PUFA | 947.1 ± 47.4 | 56.6 | 1134.4 ± 33.2 | 55.9 |
| n-3/n-6 | 0.03 | 0.19 | ||
| Biomarker | Regular Diet | D. vlkianum-Supplemented | ||||
|---|---|---|---|---|---|---|
| D16 | D30 | D66 | D16 | D30 | D66 | |
| Glycaemia 1 (mg/dL) | 72.4 ± 2.3 | 64.6 ± 1.1 | 68.7 ± 2.2 | 66.5 ± 2.7 | 60.2 ± 1.9 | 61.4 ± 2.4 |
| Total Cholesterol (mg/dL) | 47.0 ± 3.1 | 48.0 ± 3.9 | 58.2 ± 4.2 | 48.0 ± 4.0 | 48.0 ± 4.4 | 48.6 ± 1.3 # |
| HDL-C (mg/dL) | 6.8 ± 0.9 | 9.3 ± 2.8 | 14.0 ± 1.1 ** | 6.3 ± 1.0 | 9.8 ± 1.3 | 12.1 ± 0.5 ** |
| LDL-C (mg/dL) | 26.6 ± 5.5 | 23.6 ± 2.9 | 28.0 ± 3.3 | 28.6 ± 3.3 | 23.8 ± 4.7 | 19.3 ± 1.7 *,# |
| VLDL-C (mg/dL) | 13.7 ± 2.9 | 15.2 ± 1.1 | 16.2 ± 1.2 | 13.2 ± 2.3 | 14.4 ± 1.0 | 17.2 ± 1.3 |
| TAG (mg/dL) | 68.5 ± 14.6 | 76.0 ± 5.4 | 81.0 ± 6.0 | 65.8 ± 11.5 | 72.5 ± 4.8 | 85.9 ± 6.7 |
| Total Lipids (mg/dL) | 291.8 ± 16.9 | 302.8 ± 10.8 | 320.9 ± 12.8 | 289.2 ± 16.6 | 298.2 ± 8.2 | 316.6 ± 9.4 |
| Erythrocytes (% Hemolysis 67mM) | 31.0 ± 2.4 | 27.0 ± 5.5 | 29.4 ± 2.5 | 32.8 ± 1.8 | 23.3 ± 3.9 | 22.1 ± 0.3 ## |
| Creatinine (mg/dL) | 0.59 ± 0.02 | 0.49 ± 0.01 | 0.48 ± 0.02 | 0.50 ± 0.02 | 0.47 ± 0.03 | 0.47 ± 0.01 |
| Urea (mg/dL) | 34.5 ± 4.7 | 31.3 ± 0.5 | 31.2 ± 1.2 | 28.8 ± 1.7 | 33.8 ± 1.9 | 35.6 ± 2.4 |
| AST (U/L) | 119.0 ± 11.2 | 113.5 ± 10.5 | 113.5 ± 14.4 | 130.0 ± 27.1 | 99.5 ± 9.2 | 108.1 ± 8.6 |
| ALT (U/L) | 8.0 ± 0.9 | 8.3 ± 1.2 | 7.2 ± 1.1 | 5.3 ± 1.0 | 5.0 ± 0.4 | 6.3 ± 1.0 |
| ALP (U/L) | 59.3 ± 5.3 | 71.3 ± 9.5 | 57.2 ± 4.3 | 68.3 ± 10.6 | 81.3 ± 3.7 | 68.6 ± 6.8 |
| Erythrocytes | Liver | Heart | Kidney | Subcutaneous Fat | Visceral Fat | Brain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acids | CG | MG | CG | MG | CG | MG | CG | MG | CG | MG | CG | MG | CG | MG |
| 14:0 | 0.26 ± 0.04 | 0.30 ± 0.02 | 0.35 ± 0.12 | 0.34 ± 0.04 | 0.34 ± 0.17 | 0.39 ± 0.11 | 1.89 ± 0.20 | 2.18 ± 0.26 * | 1.99 ± 0.32 | 2.21 ± 0.25 | 2.23 ± 0.10 | 2.38 ± 0.12 | 0.12 ± 0.03 | 0.12 ± 0.03 |
| 16:0 | 24.3 ± 0.72 | 24.3 ± 0.63 | 19.7 ± 0.96 | 19.8 ± 0.76 | 14.8 ± 2.73 | 14.8 ± 4.02 | 27.7 ± 1.36 | 28.9 ± 1.51 | 27.0 ± 1.80 | 27.2 ± 1.25 | 29.9 ± 0.95 | 30.8 ± 2.26 | 13.6 ± 0.96 | 13.1 ± 1.01 |
| 18:0 | 12.9 ± 0.94 | 13.0 ± 0.62 | 16.3 ± 1.13 | 17.1 ± 1.40 | 15.6 ± 2.51 | 15.7 ± 0.69 | 7.76 ± 1.02 | 7.23 ± 0.56 | 2.84 ± 0.48 | 2.84 ± 0.23 | 2.31 ± 0.65 | 2.75 ± 0.39 | 16.7 ± 0.73 | 16.8 ± 0.88 |
| Other SFA | 3.20 ± 0.56 | 3.14 ± 0.37 | 0.30 ± 0.02 | 0.29 ± 0.03 | 1.41 ± 1.05 | 0.69 ± 0.44 | 1.08 ± 0.16 | 1.08 ± 0.11 | 0.49 ± 0.17 | 0.42 ± 0.07 | 0.54 ± 0.13 | 0.59 ± 0.10 | 3.50 ± 0.94 | 4.07 ± 0.52 |
| Σ SFA | 40.4 ± 0.58 | 40.5 ± 0.54 | 36.6 ± 0.73 | 37.5 ± 0.77 | 32.3 ± 2.85 | 30.4 ± 2.30 | 38.4 ± 1.50 | 39.4 ± 1.86 | 32.7 ± 3.05 | 32,5 ± 1.51 | 34.6 ± 1.02 | 35.4 ± 1.30 | 33.9 ± 1.03 | 34.1 ± 1.08 |
| 16:1 | 1.18 ± 0.34 | 1.29 ± 0.22 | 2.77 ± 1.21 | 2.70 ± 0.62 | 1.97 ± 1.12 | 1.56 ± 0.33 | 8.81 ± 1.10 | 9.27 ± 0.97 | 7.83 ± 2.58 | 7.44 ± 1.67 | 10.0 ± 2.01 | 9.55 ± 1.50 | 0.43 ± 0.11 | 0.48 ± 0.04 |
| 18:1n-9 | 5.74 ± 0.50 | 6.05 ± 0.51 | 6.19 ± 0.96 | 5.70 ± 0.50 | 5.99 ± 1.64 | 6.09 ± 1.01 | 22.9 ± 2.12 | 21.7 ± 1.16 | 22.7 ± 1.87 | 21.3 ± 1.33 | 23.4 ± 1.77 | 21.9 ± 1.50 | 26.5 ± 0.57 | 26.9 ± 1.05 |
| 18:1n-7 | 3.29 ± 0.28 | 3.50 ± 0.49 | 4.53 ± 0.66 | 4.18 ± 0.93 | 4.67 ± 0.54 | 4.75 ± 1.09 | 3.62 ± 0.29 | 3.42 ± 0.26 | 4.58 ± 0.38 | 4.38 ± 0.47 | 4.33 ± 0.38 | 4.12 ± 0.23 | nd | nd |
| Other MUFA | 0.76 ± 0.16 | 0.71 ± 0.07 | 0.39 ± 0.20 | 0.38 ± 0.04 | 0.33 ± 0.13 | 0.31 ± 0.05 | 0.44 ± 0.13 | 0.43 ± 0.09 | 0.97 ± 0.37 | 0.96 ± 0.17 | 0.80 ± 0.12 | 0.87 ± 0.15 | 9.77 ± 0.74 | 11.2 ± 1.96 |
| Σ MUFA | 11.6 ± 0.93 | 12.0 ± 0.78 | 13.9 ± 2.67 | 13.0 ± 1.86 | 12.6 ± 2.60 | 12.8 ± 2.00 | 35.8 ± 2.85 | 34.8 ± 1.66 | 36.1 ± 3.87 | 34.1 ± 2.46 | 38.6 ± 3.90 | 36.4 ± 2.62 | 37.7 ± 2.26 | 38.5 ± 2.51 |
| 18:2n-6 (LA) | 10.1 ± 0.46 | 10.5 ± 0.68 | 16.0 ± 1.35 | 16.1 ± 0.93 | 24.8 ± 2.71 | 26.4 ± 3.40 | 14.8 ± 2.54 | 15.2 ± 1.88 | 26.7 ± 4.01 | 27.5 ± 1.61 | 21.9 ± 3.36 | 22.3 ± 1.68 | nd | nd |
| 18:3n-3 (ALA) | 0.06 ± 0.03 | 0.09 ± 0.01 | 0.16 ± 0.13 | 0.16 ± 0.02 | nd | nd | 0.60 ± 0.09 | 0.70 ± 0.13 | 1.33 ± 0.09 | 1.49 ± 0.06 | 1.25 ± 0.11 | 1.34 ± 0.13 | nd | nd |
| 20:4n-6 (AA) | 23.0 ± 1.03 | 20.5 ± 0.80 * | 20.5 ± 1.86 | 18.7 ± 0.71 * | 14.2 ± 2.38 | 14.0 ± 1.23 | 6.95 ± 1.86 | 6.23 ± 0.76 | 0.69 ± 0.30 | 0.66 ± 0.27 | 0.59 ± 0.75 | 0.35 ± 0.06 | 7.13 ± 0.30 | 6.88 ± 0.71 |
| 20:5n-3 (EPA) | 0.59 ± 0.07 | 1.32 ± 0.11 * | 0.93 ± 0.14 | 2.11 ± 0.19 * | 0.19 ± 0.04 | 0.50 ± 0.12 * | 0.12 ± 0.07 | 0.19 ± 0.16 | 0.06 ± 0.05 | 0.21 ± 0.04 * | 0.04 ± 0.03 | 0.22 ± 0.07 * | nd | nd |
| 22:5n-3 (DPA) | 1.65 ± 0.16 | 2.32 ± 0.10 * | 1.17 ± 0.28 | 2.05 ± 0.25 * | 1.26 ± 0.29 | 1.48 ± 0.58 | 0.26 ± 0.08 | 0.26 ± 0.08 | 0.12 ± 0.11 | 0.18 ± 0.14 | 0.05 ± 0.08 | 0.14 ± 0.10 | nd | nd |
| 22:6n-3 (DHA) | 3.84 ± 0.25 | 3.73 ± 0.25 | 6.65 ± 0.89 | 6.44 ± 0.42 | 7.47 ± 1.83 | 7.94 ± 0.97 | 0.68 ± 0.20 | 0.70 ± 0.11 | 0.39 ± 0.14 | 0.47 ± 0.06 | 0.22 ± 0.14 | 0.40 ± 0.27 | 10.9 ± 0.99 | 10.4 ± 1.00 |
| Other PUFA | 1.66 ± 0.27 | 1.63 ± 0.09 | 1.32 ± 0.42 | 1.62 ± 0.11 | 1.46 ± 0.34 | 1.85 ± 0.77 | 1.45 ± 0.06 | 1.56 ± 0.27 | 1.92 ± 0.67 | 1.72 ± 0.46 | 1.72 ± 0.93 | 1.63 ± 0.83 | 8.67 ± 0.37 | 8.87 ± 0.65 |
| Σ PUFA | 43.2 ± 1.37 | 42.6 ± 1.05 | 46.6 ± 2.70 | 47.0 ± 1.27 | 49.3 ± 5.92 | 52.4 ± 2.04 | 24.6 ± 2.07 | 24.7 ± 1.94 | 30.1 ± 4.62 | 31.4 ± 1.72 | 25.4 ± 4.20 | 25.9 ± 1.79 | 26.7 ± 1.12 | 26.2 ± 1.39 |
| Σ n-3 | 9.05 ± 0.28 | 10.4 ± 0.25 | 9.39 ± 1.11 | 11.2 ± 0.51 * | 9.10 ± 1.80 | 11.5 ± 1.19 * | 1.82 ± 0.26 | 2.02 ± 0.35 | 1.97 ± 0.47 | 2.48 ± 0.21 | 1.87 ± 0.50 | 2.05 ± 0.41 | 17.9 ± 0.81 | 17.7 ± 0.72 |
| Σ n-6 | 33.7 ± 1.31 | 31.6 ± 0.99 | 37.0 ± 2.93 | 35.5 ± 1.42 | 39,2 ± 4.52 | 40.2 ± 2.87 | 21.8 ± 2.77 | 21.5 ± 1.90 | 27.7 ± 4.34 | 28.5 ± 1.71 | 23.0 ± 3.88 | 23.4 ± 1.74 | 8.53 ± 0.48 | 8.22 ± 0.76 |
| Σ n-3/Σn-6 | 0.27 ± 0.01 | 0.33 ± 0.01 * | 0.26 ± 0.04 | 0.32 ± 0.02 * | 0.23 ± 0.04 | 0.29 ± 0.05 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 * | 0.08 ± 0.02 | 0.09 ± 0.02 | 2.10 ± 0.10 | 2.16 ± 0.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mello-Sampayo, C.; Paterna, A.; Polizzi, A.; Duarte, D.; Batista, I.; Pinto, R.; Gonçalves, P.; Raymundo, A.; Batista, A.P.; Gouveia, L.; et al. Evaluation of Marine Microalga Diacronema vlkianum Biomass Fatty Acid Assimilation in Wistar Rats. Molecules 2017, 22, 1097. https://doi.org/10.3390/molecules22071097
De Mello-Sampayo C, Paterna A, Polizzi A, Duarte D, Batista I, Pinto R, Gonçalves P, Raymundo A, Batista AP, Gouveia L, et al. Evaluation of Marine Microalga Diacronema vlkianum Biomass Fatty Acid Assimilation in Wistar Rats. Molecules. 2017; 22(7):1097. https://doi.org/10.3390/molecules22071097
Chicago/Turabian StyleDe Mello-Sampayo, Cristina, Angela Paterna, Ambra Polizzi, Diana Duarte, Irineu Batista, Rui Pinto, Patrícia Gonçalves, Anabela Raymundo, Ana P. Batista, Luísa Gouveia, and et al. 2017. "Evaluation of Marine Microalga Diacronema vlkianum Biomass Fatty Acid Assimilation in Wistar Rats" Molecules 22, no. 7: 1097. https://doi.org/10.3390/molecules22071097