Solar or UVA-Visible Photocatalytic Ozonation of Water Contaminants
Abstract
:1. Introduction
2. The Solar Photocatalytic Ozonation Process
2.1. Radiation Sources and Photoreactors Used
2.2. Catalysts Used
2.3. Catalyst Synthesis and Characterization
2.4. Organics Treated
2.5. Influence of Main Variables
2.6. Intermediates, Ozone Consumption, Biodegradability, and Toxicity
2.7. Mechanism and Kinetics: The Use of Scavengers
2.8. Stability and Activity of Catalysts
3. Conclusions and Future Steps
Acknowledgments
Conflicts of Interest
References
- Navarro, S.; Vela, N.; Gimenez, M.J.; Navarro, G. Persistence of four s-triazine herbicides in river, sea and groundwater samples exposed to sunlight and darkness under laboratory conditions. Sci. Total Environ. 2004, 329, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, H. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2011, 416, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, P.; Grenni, P.; Lucentini, L.; Barra Caracciolo, A. Terbuthylazine and other triazines in Italian water resources. Microchem. J. 2013, 107, 136–142. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.E.; Boxall, A.B.A.; Brown, A.R.; Cuthbert, R.J.; Gaw, S.; Hutchinson, T.H.; Jobling, S.; Madden, J.C.; Metcalfe, C.D.; Naidoo, V.; et al. Assessing the exposure risk and impacts of pharmaceuticals in the environment on individuals and ecosystems. Biol. Lett. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of data constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Beltrán, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press (Lewis Publishers): Boca Ratón, FL, USA, 2004. [Google Scholar]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F. Mechanism and kinetics of sulfamethoxazole photocatalytic ozonation in water. Water Res. 2009, 43, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.J.; Sagasti, J.; Encinas, A.; Gimeno, O. Contaminants abatement by ozone in secondary effluents. Evaluation of second-order rate constants. J. Chem. Technol. Biotechnol. 2011, 86, 1058–1066. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.Y.; von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Dodd, M.C.; Buffle, M.O.; von Gunten, U. Oxidation of antibacterial molecules by aqueous ozone: moiety-specific reaction kinetics and application to ozone-based wastewater treatment. Environ. Sci. Technol. 2006, 40, 6519–6530. [Google Scholar] [CrossRef]
- Jin, X.; Peldszus, S.; Huck, P.M. Reaction kinetics of selected micropollutants in ozonation and advanced oxidation processes. Water Res. 2012, 46, 6519–6530. [Google Scholar] [CrossRef] [PubMed]
- Deborde, M.; Rabouan, S.; Duguet, J.P.; Legube, B. Kinetics of aqueous ozone-induced oxidation of some endocrine disruptors. Environ. Sci. Technol. 2005, 39, 6086–6092. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Adams, C.; Surampalli, R. Determination of ozonation rate constants for lincomycin and spectinomycin. Ozone Sci. Eng. 2004, 26, 526–537. [Google Scholar] [CrossRef]
- Staehelin, S.; Hoigné, J. Decomposition of ozone in water: Rate of initiation by hydroxyde ions and hydrogen peroxide. Environ. Sci. Technol. 1982, 16, 666–681. [Google Scholar] [CrossRef]
- Staehelin, S.; Hoigné, J. Decomposition of Ozone in Water the Presence of Organic Solutes Acting as Promoters and Inhibitors of Radical Chain Reactions. Environ. Sci. Technol. 1985, 19, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Ziolek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Ikehata, K.; Naghashkar, N.J.; El-Din, M.G. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.P.; Wu, D.; Zhang, Y. Heterogeneous Catalytic Ozonation Reaction Mechanism. Prog. Chem. 2016, 28, 1112–1120. [Google Scholar]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds—A review. Chem. Eng. J. 2017, 310, 437–456. [Google Scholar] [CrossRef]
- Noufi, R.N.; Kohl, P.A.; Frank, S.N.; Bard, A.J. Semiconductor electrodes XIV. Electrochemistry and electroluminescence at n-type TiO2 in aqueous solutions. J. Electrochem. 1978, 125, 246–252. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Photocatalysis and Water Purification; Pichat, P. (Ed.) Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Photocatalysis: Fundamentals, Materials and Potential; Pichat, P. (Ed.) MDPI: Basel, Switzerland, 2016. [Google Scholar]
- Schneider, J.; Bahnemann, D.; Ye, J.; Puma, L.G.; Dionysiou, D.D. (Eds.) Photocatalysis, Vol. 1: Fundamentals and Perspectives; The Royal Society of Chemistry (RSC): London, UK, 2016. [Google Scholar]
- Pichat, P. Fundamentals of TiO2 photocatalysis. Consequences for some environmental applications. In Heterogeneous Photocatalysis; Colmenares Quintero, J.C., Xu, Y.-J., Eds.; Springer: Basel, Switzerland, 2016; pp. 321–359. [Google Scholar]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Malato, S.; Fernandez-Ibañez, P.; Maldonado, M.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170, 90–123. [Google Scholar] [CrossRef]
- Hubner, U.; von Gunten, U.; Jekel, M. Evaluation of the persistence of transformation products from ozonation of trace organic compounds—A critical review. Water Res. 2015, 68, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Rakovsky, S.K.; Anachkov, M.P.; Iliev, V.I.; Elyias, A.E. Ozone Degradation of Alcohols, Ketones, Ethers and Hydroxybenzenes: Determination of Pathways and Kinetic Parameters. J. Adv. Oxid. Technol. 2013, 31–51. [Google Scholar] [CrossRef]
- Agustina, T.E.; Ang, H.M.; Vareek, V.K. A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 264–273. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Muller, S.; Moller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Cao, H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere 2015, 121, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Radwan, E.K.; Yu, L.L.; Achari, G.; Langford, C.H. Photocatalytic ozonation of pesticides in a fixed bed flow through UVA-LED photoreactor. Environ. Sci. Pollut. Res. 2016, 23, 21313–21318. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Yanagisawa, I.; Takeuchi, M.; Koike, T.; Serpone, N.; Hidaka, H. Remediation of simulated aquatic sites contaminated with recalcitrant substrates by TiO2/ozonation under natural sunlight. Appl. Catal. B Environ. 2009, 91, 242–246. [Google Scholar] [CrossRef]
- Araña, J.; Herrer, J.A.; Doña, J.M.; González, O. TiO2-photocatalysis as a tertiary treatment of naturally treated wastewater. Catal. Today 2002, 76, 279–289. [Google Scholar] [CrossRef]
- Bayarri, B.; Gonzalez, O.; Maldonado, M.I.; Gimenez, J.; Esplugas, S. Comparative study of 2,4-dichlorophenol degradation with different advanced oxidation processes. J. Sol. Energy Eng. Trans. ASME 2007, 129, 60–67. [Google Scholar] [CrossRef]
- Nishimoto, S.; Mano, T.; Kameshima, Y.; Miyake, M. Photocatalytic water treatment over WO3 under visible light irradiation combined with ozonation. Chem. Phys. Lett. 2010, 500, 86–89. [Google Scholar] [CrossRef]
- Anandan, S.; Lee, G.J.; Chen, P.K.; Fan, C.; Wu, J.J. Removal of Orange II Dye in Water by Visible Light Assisted Photocatalytic Ozonation Using Bi2O3 and Au/Bi2O3 Nanorods. Ind. Eng. Chem. Res. 2010, 49, 9729–9737. [Google Scholar] [CrossRef]
- Oyama, T.; Otsu, T.; Hidano, Y.; Koike, T.; Serpone, N.; Hidaka, H. Enhanced remediation of simulated wastewaters contaminated with 2-chlorophenol and other aquatic pollutants by TiO2-photoassisted ozonation in a sunlight-driven pilot-plant scale photoreactor. Sol. Energy 2011, 85, 938–944. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, M.H.; Yu, L.E. Hydrothermal synthesis and photocatalytic performance of metal-ions doped TiO2. Appl. Catal. A Gen. 2012, 413–414, 238–244. [Google Scholar] [CrossRef]
- Rey, A.; Quiñones, D.H.; Alvarez, P.M.; Beltran, F.J.; Plucinski, P.K. Simulated solar-light assisted photocatalytic ozonation of metoprolol over titania-coated magnetic activated carbon. Appl. Catal. B Environ. 2012, 111, 246–253. [Google Scholar] [CrossRef]
- Shin, D.; Jang, M.; Cui, M.; Na, S.; Khim, J. Enhanced removal of dichloroacetonitrile from drinking water by the combination of solar-photocatalysis and ozonation. Chemosphere 2013, 93, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.Z.; Zhu, D.; Li, L.; Lan, B. Enhanced Photocatalytic Ozonation of Organics by g-C3N4 Under Visible Light Irradiation. J. Hazard. Mater. 2014, 280, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, D.H.; Rey, A.; Alvarez, P.M.; Beltran, F.J.; Plucinski, P.K. Enhanced activity and reusability of TiO2 loaded magnetic activated carbon for solar photocatalytic ozonation. Appl. Catal. B Environ. 2014, 144, 96–106. [Google Scholar] [CrossRef]
- Marquez, G.; Rodriguez, E.M.; Beltran, F.J.; Alvarez, P.M. Solar photocatalytic ozonation of a mixture of pharmaceutical compounds in water. Chemosphere 2014, 113, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; García-Muñoz, P.; Hernández-Alonso, M.D.; Mena, E.; García-Rodríguez, S.; Beltrán, F.J. WO3–TiO2 based catalysts for the simulated solar radiation assisted photocatalytic ozonation of emerging contaminants in a municipal wastewater treatment plant effluent. Appl. Catal. B Environ. 2014, 154–155, 274–284. [Google Scholar] [CrossRef]
- Mano, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Water treatment efficacy of various metal oxide semiconductors for photocatalytic ozonation under UV and visible light irradiation. Chem. Eng. J. 2015, 264, 221–229. [Google Scholar] [CrossRef]
- Rey, A.; Mena, E.; Chávez, A.M.; Beltrán, F.J.; Medina, F. Influence of structural properties on the activity of WO3 catalysts for visible light photocatalytic ozonation. Chem. Eng. Sci. 2015, 126, 80–90. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Contreras, S.; Beltrán, F.J. Visible light photocatalytic ozonation of DEET in the presence of different forms of WO3. Catal. Today 2015, 252, 100–106. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Alvarez, P.M.; Rey, A.; Contreras, S.; Beltran, F.J. Application of solar photocatalytic ozonation for the degradation of emerging contaminants in water in a pilot plant. Chem. Eng. J. 2015, 260, 399–410. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Alvarez, P.M.; Rey, A.; Beltrán, F.J. Removal of emerging contaminants from municipal WWTP secondary effluents by solar photocatalytic ozonation. A pilot-scale study. Separat. Purific. Technol. 2015, 149, 132–139. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Rey, A.; Alvarez, P.M.; Beltran, F.J.; Puma, G.L. Boron doped TiO2 catalysts for photocatalytic ozonation of aqueous mixtures of common pesticides: Diuron, o-phenylphenol, MCPA and terbuthylazine. Appl. Catal. B Environ. 2015, 178, 74–81. [Google Scholar] [CrossRef]
- Gimeno, O.; Garcia-Araya, J.F.; Beltran, F.J.; Rivas, F.J.; Espejo, A. Removal of emerging contaminants from a primary effluent of municipal wastewater by means of sequential biological degradation-solar photocatalytic oxidation processes. Chem. Eng. J. 2016, 290, 12–20. [Google Scholar] [CrossRef]
- Liao, G.; Zhu, D.; Li, C.; Lan, B.; Li, L. Degradation of Oxalic Acid and Bisphenol A by Photocatalytic Ozonation with g-C3N4 Nanosheet under Simulated Solar Irradiation. Ozone Sci. Eng. 2016, 38, 312–317. [Google Scholar] [CrossRef]
- Yin, J.; Liao, G.; Zhu, D.; Lu, P.; Li, L. Photocatalytic ozonation of oxalic acid by g-C3N4/graphene composites under simulated solar irradiation. J. Photochem. Photobiol. A Chem. 2016, 315, 138–144. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Fourie, C.J.S.; Momba, M.N.B. Synergistic effect of UV-vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: A comparative study. J. Catal. 2016, 341, 116–125. [Google Scholar] [CrossRef]
- Alvarez, P.M.; Quiñones, D.H.; Terrones, I.; Rey, A.; Beltran, F.J. Insights into the removal of terbuthylazine from aqueous solution by several treatment methods. Water Res. 2016, 98, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Rey, A.; Rodriguez, E.M.; Beltran, F.J. Nanostructured CeO2 as catalysts for different AOPs based in the application of ozone and simulated solar radiation. Catal. Today 2017, 280, 74–79. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Rodríguez, E.M.; Beltrán, F.J. Reaction mechanism and kinetics of DEET visible light assisted photocatalytic ozonation with WO3 catalyst. Appl. Catal. B Environ. 2017, 202, 460–472. [Google Scholar] [CrossRef]
- Beltrán, F.J.; García-Araya, J.F.; Giráldez, I. Gallic acid water ozonation using activated carbon. Appl. Catal. B Environ. 2006, 63, 249–259. [Google Scholar] [CrossRef]
- Tomiyasu, H.; Fukutomi, H.; Gordon, G. Kinetics and mechanism of ozone decomposition in basic aqueous solutions. Inorg. Chem. 1985, 24, 2962–2966. [Google Scholar] [CrossRef]
- Bidga, R.J. Considering Fenton’s chemistry for wastewater treatment. Chem. Eng. Prog. 1995, 91, 62–67. [Google Scholar]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Begum, N.S.; Ahmed, H.M.F.; Hussain, O.M. Characterization and photocatalytic activity of boron-doped TiO2 thin films prepared by liquid phase deposition technique. Bull. Mater. Sci. 2008, 31, 741–745. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Kudo, A. Synthesis and photocatalytic performances of BiVO4 by ammonia co-precipitation process. J. Solid State Chem. 2009, 182, 223–228. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010. [Google Scholar]
- Calzadilla, A.; Rehdanz, K.; Tol, R. The economic impact of more sustainable water use in agriculture: A computable general equilibrium analysis. J. Hydrol. 2010, 384, 292–305. [Google Scholar] [CrossRef]
- Fukazawa, H.; Hoshino, K.; Shiozawa, T.; Matsushita, H.; Terao, Y. Identification and quantification of chlorinated bisphenol-A in wastewater from wastepaper recycling plants. Chemosphere 2001, 44, 973–979. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Gimeno, O.; Rivas, F.J.; Carbajo, M. Photocatalytic Ozonation of Gallic Acid in Water. J. Chem. Technol. Biotechnol. 2006, 81, 1787–1796. [Google Scholar] [CrossRef]
- Taube, H. Photochemical reactions of ozone in aqueous solution. Trans. Faraday Soc. 1957, 53, 656–665. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Rate constants of the reactions of ozone with organic and inorganic compounds. II. Dissociating organic compounds. Water Res. 1983, 17, 185–194. [Google Scholar] [CrossRef]
- Wang, K.; Hsieh, Y.; Chou, M.; Chang, C. Photocatalytic degradation of 2-chloro and 2-nitrophenol by titanium dioxide suspensions in aqueous solution. Appl. Catal. B Environ. 1999, 22, 1–8. [Google Scholar] [CrossRef]
- Doong, R.; Chen, C.; Maithreepala, R.; Chang, S. The influence of pH and cadmium sulfide on the photocatalytic degradation of 2-chlorophenol in titanium dioxide suspensions. Water Res. 2001, 35, 2880–2973. [Google Scholar] [CrossRef]
- Delanghe, B.; Mekras, C.I.; Graham, N.J.D. Aqueous ozonation of surfactants—A review. Ozone Sci. Eng. 1991, 13, 639–673. [Google Scholar] [CrossRef]
- Alvares, A.B.C.; Diaper, C.; Parsons, S.A. Partial oxidation by ozone to remove recalcitrance from wastewaters—A review. Environ. Technol. 2001, 22, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Collado, N.; Buttiglierib, G.; Martib, E.; Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D.; Comasa, J.; Rodriguez-Roda, L. Effects on activated sludge bacterial community exposed to sulfamethoxazole. Chemosphere 2013, 93, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Márquez, G.; Rodríguez, E.M.; Maldonado, M.I.; Álvarez, P.M. Integration of ozone and solar TiO2-photocatalytic oxidation for the degradation of selected pharmaceutical compounds in water and wastewater. Sep. Purif. Technol. 2014, 136, 18–26. [Google Scholar] [CrossRef]
- Turchi, S.C.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Jenks, W.S. Photocatalytic reactions pathways—Effects of molecular structure, catalyst and wavelength. In Photocatalysis and Water Purification; Pichat, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 25–51. [Google Scholar]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F. Kinetic modelling of TOC removal in the photocatalytic ozonation of diclofenac aqueous solutions. Appl. Catal. B Environ. 2010, 100, 289–298. [Google Scholar] [CrossRef]
- Pichat, P.; Disdier, J.; Hoang-Van, C.; Mas, D.; Goutailler, G.; Gaysse, C. Purification/deodorization of indoor air and gaseous effluents by TiO2 photocatalysis. Catal. Today 2000, 63, 363–369. [Google Scholar] [CrossRef]
- Ross, F.; Ross, A.B. Selected Specific Rates of Reactions of Transients for Water in Aqueous Solution. III Hydroxyl Radical and Perhydroxyl Radical and Their Radical Ions; US National Bureau of Standards: Washington, DC, USA, 1977.
- Sawyer, D.T.; Valentine, J.S. How super is superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Yao, D.C.C.; Haag, W.R. Rate constants for direct reactions of ozone with several drinking water contaminants. Water Res. 1991, 25, 761–773. [Google Scholar]
- Elovitz, M.S.; von Gunten, U. Hydroxyl radical/ozone ratios during ozonation processes. I. The Rct concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
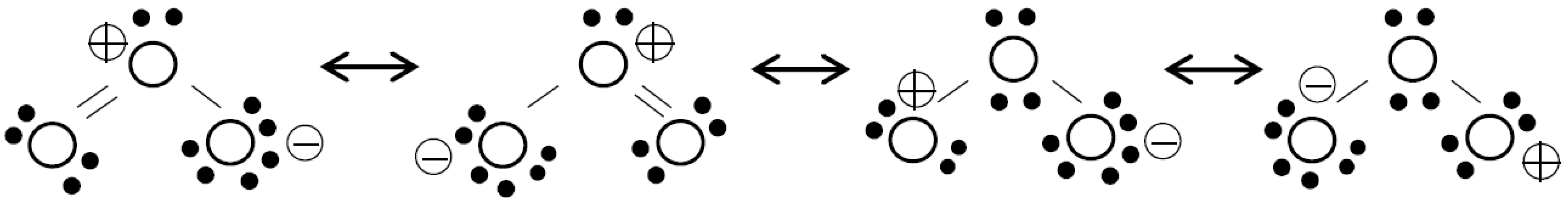
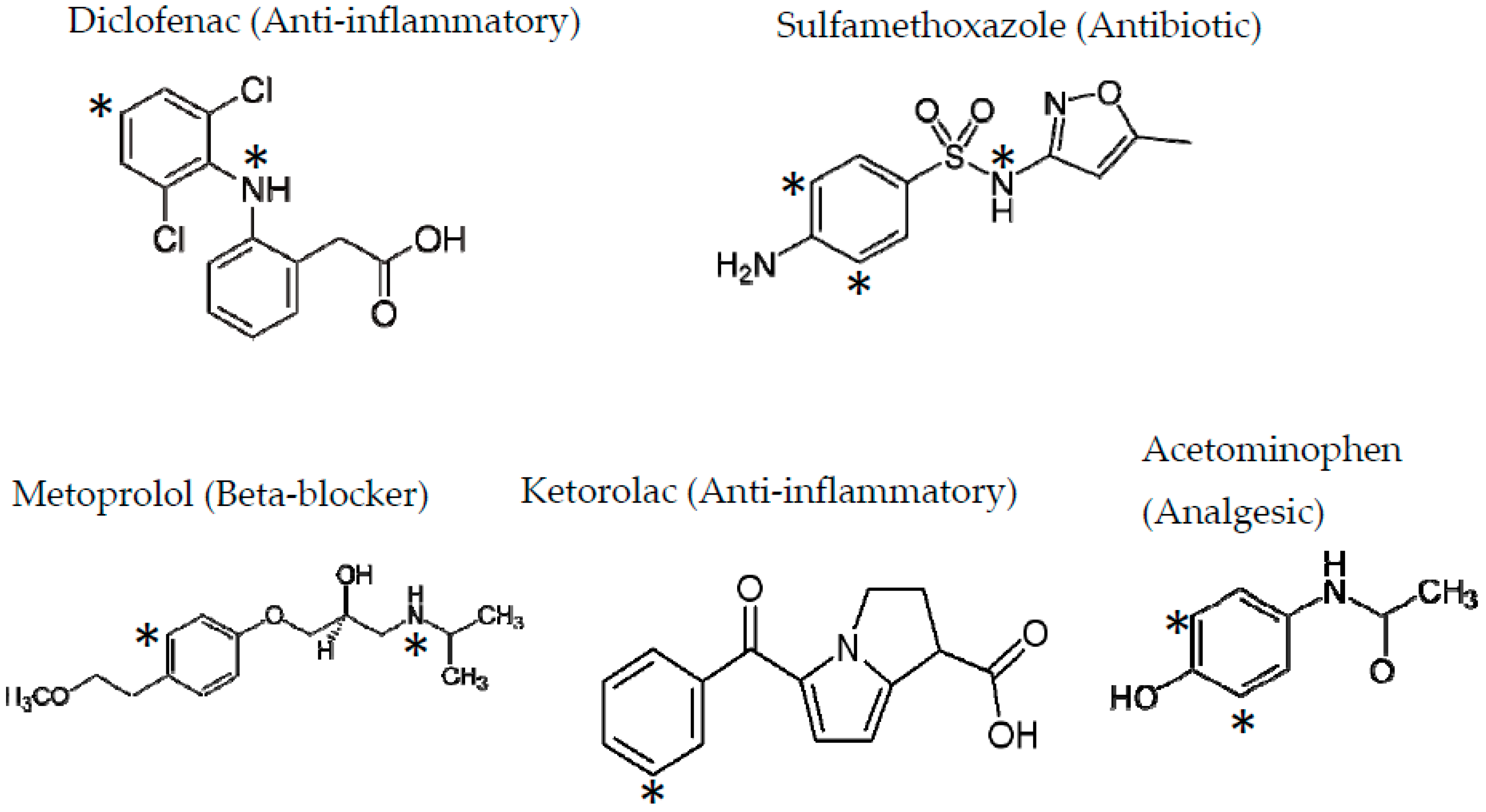
| Compound | Activity | Rate Constant (M−1 s−1) | Reference |
|---|---|---|---|
| Sulfamethoxazole | Antibiotic | 5.5 × 105 | [9] |
| Diclofenac | Anti-inflammatory | 105 | [10] |
| Ketorolac | Analgesic | 3.4 × 105 | |
| Acetaminophen | Anti-inflammatory | 2.7 × 105 | |
| Metoprolol | Beta-blocker | 2.5 × 103 | |
| Carbamacepine | Analgesic | 3 × 105 | [11] |
| 17α-ethinylestradiol | Hormone | 3 × 106 | |
| Tetracycline | Antibiotic | 1.9 × 106 | [12] |
| Fenoterol | Breath aider | 2.8 × 106 | [13] |
| Gemfibrozil | Lipid regulator | 4.9 × 105 | |
| Estriol | Hormone | 105 | [14] |
| Lyncomycine | Antibiotic | 6.7 × 105 | [15] |
| Compounds Treated and Processes Applied | Data on Catalysts, Ozone, and Others | Radiation Source | Photoreactor Type | Observations | Ref. |
|---|---|---|---|---|---|
| Effluent from an urban wastewater lagoon. Total coliforms followed solar photolysis photocatalytic oxidation (SPO). | P25 TiO2 alone: 2 g L−1. Activated carbon (AC) alone: 0.3 g/L. Mixture TiO2-AC: 2.3 g L−1. Ozone dose or concentration: not given. | Solar light: 10 to 14 h radiation. | Agitated or bubble vessels of 250 mL. | COD removal: 40% with SPO (best result) and AC-TiO2. Total coliforms removal: >99.99%. Inorganic ions determined: NO2−, PO43−. Fourier Transform FTR study. | [41] |
| 2,4 dichlorophenol (DCP) at 100 mg L−1. H2O2 concentration: 250 and 75 mg/L in UV/H2O2 and UV/Fe/H2O2, respectively. O3 + Fe(II) + UV, photo-Fenton, UV + Fe(III), UV + H2O2, photocatalysis and photolysis. | Two catalysts: 1. Fe(III): 10–30 mg L−1 as FeCl3. 2. TiO2: 0.5 g L−1. Ozone: 5.5 g h−1. | 1. Blue UVA lamp (350 nm); 2.5 μE s−1. 2. Solar simulator: 6 μE s−1. 3. The Sun: 236 μE s−1. 4. Eight 15 W UVA blue fluorescent lamps (300–420 nm): 120 μE s−1 (μE s−1: μEinstein s−1). | 1. UV, UV + H2O2, UV + Fe(II), Photo-Fenton runs in 12 × 13 cm photoreactor. 2. Solar simulator with parabolic mirrors and quartz tube reactor. 3. Three compound parabolic collectors CPC, each with eight tubes in series. 4. One 2 L gas-liquid contactor + tube photoreactor. | Scaling up factors for scaling up to pilot-plant size. Estimation of amount of waste water that could be treated. Analysis: DCP concentration and TOC. DCP degradation is plotted as a function of accumulated photons per liter entering the reactor. Apparent pseudo first order kinetics. Pseudo quantum yield as a function of incident energy. | [42] |
| 2,4-dichlorophenoxyacetic acid, bisphenol A, Sodium butylnaphthalenesulfonate and benzyldodecyldimethyl-ammonium bromide surfactants. 2 mM concentration: 100, 175 and 250 mg L−1. Processes: O3, O3/UV, O2/UV/TiO2 and O3/UV/TiO2. | P25 TiO2 at 2 g L−1. | One 75 W High Pressure Hg lamp (3 mW cm−2 at 360 nm) and the Sun. | Twenty Pyrex glass tubular reactors. | TOC measurement. | [40] |
| Phenol: 0.169 mg L−1. Processes: O3, O3/Vis, O2/Vis/cat, O3/cat and O3/Vis/cat. Ozone process: 0.45 g h−1. | Commercial WO3 powder and n-TiO2: 0.2 g L−1. | 300 W Xe lamp with a cut-off filter (k > 420 nm). | Pyrex inner-irradiation vessel placed in a water bath set at room temperature. | Catalyst characterization: SEM, XRD, SBET. AOP comparison results for compound concentration and TOC. Repetitive experiments for catalyst reuse. | [43] |
| Orange II dye: 10−4 M. Processes: O3 100 mL/min and 2 mg L−1 of ozone in influent gas. Processes: O3, O3 cat, O2/UV/cat, O3/UV/cat. | Bi2O3 and Au/Bi2O3 nanorods: 1 g L−1. | 55 W Xe lamp with 20,200 Lux and a 320 nm cut-off filter. Wavelength > 320 nm. | A 500-mL capacity borosilicate glass photoreactor with walls covered with aluminum foil to avoid the release of radiation. | Microwave and hydrothermal methods of catalyst synthesis. Catalyst characterization: DRX, SEM, TEM, XPS, EDX, DRUV-visible. Dye absorbance and concentration. Role of photosensitization. Reusability studies. Byproduct identification. | [44] |
| 2-Chlorophenol, 2,4-dichlorophenoxyacetic acid, bisphenol A, sodium dodecylbenzenesulfonate, sodium butylnaphthalenesulfonate, and benzyldodecyldimethyl- ammonium bromide surfactants at 0.5 and 0.1 mM in simulated wastewater. Processes: UV/O3, O2/TiO2/sunlight, and O3/TiO2/sunlight. 3 mg L−1 dissolved ozone. | Dispersed TiO2 P25 (0–2 g L−1) and TiO2-coated glass. | Solar light. | Pilot plant: three modules of 40 Pyrex glass tubes (inner diameter, 1.76 cm; length: 145 cm), each connected in series. Photoreactor volume: 42.3 L and total solar light harvest area of the three modules: 3.06 m2. Bubble ozone column connected in series with photoreactor modules. Solar cell for electric power to the photoreactor and ozonator. | Contaminants, chloride ion concentrations, and TOC followed vs. accumulated sunlight energy incident on the photoreactor per liter of the solution. | [45] |
| Dyes: Rhodamine B for UVA radiation and Methylene Blue for Visible radiation. At 10 mg L−1. | TiO2 and M-TiO2 catalysts. M = Ag+, Cu2+, Mn2+, Ce3+, Fe3+ and Zr4+ ions. Main catalyst used: Mn-TiO2 0.25 g L−1 (0–7% Mn). | Lamp not given; full solar radiation wavelength (300 and above) and only visible light (λ > 400 nm). | Cylindrical Pyrex vessel surrounded by a cooling water jacket in a solar simulating box. | Catalyst characterization: DRX, SBET, DRUV-Vis. Absorbance of dyes solutions followed. Correlation between SBET and dye removal percentage. | [46] |
| Metoprolol (MTP) at 10 to 50 mg L−1 Processes: O3/Light, O3/Cat/Light, and all combinations. | 1. Fe3O4/TiO2/AC 331 m2 g−1, 68% Anatase (0.38 g L−1) 2. P25 TiO2 (0.25 g L−1). Ozone gas inlet: 20 L h−1 and 6 mg L−1. | 1500 W Xe lamp with limited radiation above 300 nm with filters. 550 W/m2. | Glass-made agitated tank provided with gas inlet, gas outlet, and liquid sampling ports. | Catalyst characterization: nitrogen adsorption, XRD, FTIR, SEM, EDX, SQUID magnetometer. Reusability and activity in five cycles. MTP Concentration. TOC followed. | [47] |
| Dichloroacetonitrile at 1 ppm. Ozone: 1–1.4 g L−1 h−1. Processes: UVsolar/TiO2, O3, O3/TiO2, UVsolar/O3, and UVsolar/TiO2/O3. | P25 TiO2 ozono dosage: 1–1.38 g L−1 h−1. | Three halide lamps (100, 250, and 400 W) and the Sun with similar light spectra from 300 to 800 nm. | 1. Bench system: three halide lamps (100, 250, and 400 W), 60 cm at the top of three quartz tubes (40 cm × 2.7 cm). 2. CPC reactor (38° tilted) and cylindrical ozone reactor (1.1 m high, 10 cm internal diameter) in series. Turbulent regime. | Influence of different AOPs, pH (3, 6.5, and 10), W (4.6 to 33.8 W m−2), catalyst dosage 0.2–2.5 g L−1. Temperature: 10 to 40 °C. | [48] |
| Bisphenol A (BPA) and oxalic acid, 10 mg L−1. Ozone: 1 mL min−1 and 500 mgh−1. Processes: O3, UV-Vis/cat/O2, O3/UV-Vis/cat. | Graphitic carbon nitride (g-C3N4): Composed of numerous interconnected nanosheets, 0.5 g L−1. | High-pressure Xee long-arc lamp jacketed by a quartz thimble (GXZ500 W). Filter with Na2NO2 to cut λ < 400 nm. | One 1 L glass tubular photoreactor (8.5 × 40 cm). | Synthesis (from urea) and characterization TEM, FTIR, BET (67 m2 g−1), XRD, UV-Vis (2.7 eV, 450 nm max.). Rates for oxalic acid and BPA higher than sum of rates of single processes. Tert-butanol (TBA), triethanolamine (TEOA), and benzoquinone (BQ) act as the hydroxyl radical, hole and O2—scavenger. | [49] |
| Metoprolol, 50 ppm. Processes: O3/Light, O3/Cat/Light, and all combinations. | 1. Fe3O4/TiO2/AC 331 m2 g−1, 68%. Anatase (0.38 g L−1) 2. P25 TiO2 (0.25 g L−1). Ozone gas inlet: 20 L h−1 and 6 mg L−1. | 1500 W Xe lamp with limited radiation above 300 nm with filters. 550 Wm−2. 300–800 nm, 320–800 nm, and 390–800 nm. | Glass-made agitated tank provided with gas inlet, gas outlet, and liquid sampling ports. | Catalyst preparation and characterization (nitrogen adsorption, XRD, SEM, EDX, XPS and SQUID magnetometer). MTP concentration and TOC, dissolved O3, H2O2, acid intermediates. | [50] |
| Atenolol, Hydrochlorothiazide, Ofloxacin, and Trimethoprim in ultrapure water (10 mg L−1 doping) and WWSE (0.5 mg L−1 doping). Processes: O3, UVA-Vis, O3/cat, O3/UVA-Vis, O2/cat/UVA-Vis, O3/Cat/UVA-Vis. | P25 TiO2 45 L h−1 and 20 mg L−1 for ozone in the inlet gas. | Solar light (visible + UVA) with: 35 ± 5 W m−2. | CPC: four tubes in series, 300–400 L h−1. Water flow rate for recirculation T = 18–30 °C. | ECs concentration, TOC, ecotoxicity (Daphnia magna), Phenolic compounds formed, BOD/COD. Ozone consumption. | [51] |
| Caffeine, metoprolol, and ibuprofen: 2 mg L−1 each in Municipal Wastewater MWW Processes: O3, UVA-Vis, O3/cat, O3/UVA-Vis, O2/cat/UVA-Vis, O3/Cat/UVA-Vis. 20 mg/L O3 and 20 L h−1. | WO3/TiO2 (from P25 and titanate nanotubes) 3.8%. WO3 and 0.5 g L−1. | 1500 W Xe lamp with limited radiation restricted to wavelengths over 320 nm because of the presence of quartz, glass, and polyester cut-off filters with 550 W m−2. | 0.5L semi-batch glass-made spherical reactor, provided with a gas inlet, a gas outlet, and a liquid sampling port in a commercial solar simulator chamber. | Catalyst characterization: ICP, N2 adsorption–desorption isotherms (SBET), XRD, TEM, Raman, XPS, and DRUV-Vis spectroscopy. Contaminant concentration and TOC followed. | [52] |
| Oxalic acid: 0.01 M (TOC = 240 ppm). | TiO2P25, Nb2O5, SnO2, WO3, Fe2O3, In2O3, and BiVO4: 2 g/L. O3: 14 mg L−1 and 0.45 g h−1. | 300 W Xe lamp with an IR cut-off filter. Incident light was ca. 200 mW in the range of 360 to 470 nm. For only visible irradiation, another filter was used: λ > 410 nm. | Pyrex inner irradiation vessel placed in a thermostatic water bath. | SBET: 1.7 to 54.1 m2 g−1 TOC. Visible active properties of semiconductors (only WO3, Fe2O3, In2O3, and BiVO4). WO3: Best material for photocatalytic ozonation under visible light irradiation. | [53] |
| Ibuprofen: 10 mg L−1, and a mixture of acetaminophen, metoprolol, caffeine, hydrochlorothiazide, antipyrine, sulfamethoxazole, carbamazepine, ketorolac, diclofenac, and ibuprofen in an MWWT: 0.5 mg L−1 each Processes: adsorption, photolysis, O3, UVA-Vis, O3/cat, O3/UVA-Vis, O2/cat/UVA-Vis, O3/cat/UVA-Vis. Ozone processes: 10 mg L−1 and 20 L h−1 gas. | WO3: 0.25 g L−1. | 1500 W air-cooled Xe arc lamp with emission restricted to visible light (λ > 390 nm) because of quartz, glass, and polyester cut-off filters. 550 Wm−2. | 0.5-L glass-made spherical reactor in the chamber of a box simulator. | Preparation conditions: Calcination temperature and time. Characterization: TGA-DTA, XRD, N2 adsorption-desorption isotherms, pHPZC, XPS, and DRUV-Vis spectra. Contaminant concentrations and TOC followed. Mechanism, kinetic regime of ozonation. | [54] |
| N,N-diethyl-meta-toluamide DEET: 5 mg L−1 Processes: adsorption, photolysis, O3, UVA-Vis, O3/cat, O3/UVA-Vis, O2/cat/UVA-Vis, O3/Cat/UVA-Vis. Ozone processes: 10 mg L−1 and 15 L h−1 gas. | Commercial and homemade WO3 catalysts: 0.25 g L−1. Calcination temperature: 500 to 700 °C. | 1500 W air-cooled Xe arc lamp with emission restricted to visible light (λ > 390 nm) because of quartz, glass, and polyester cut-off filters. 550 Wm−2. | 0.5 L glass-made spherical Reactor in the chamber of a box simulator | Synthesis method. Catalyst characterization: XRD, Raman, N2 adsorption-desorption isotherms (SBET), SEM, XPS. Contaminant concentration and TOC followed. Oxalic, acetic, and formic acids were followed. | [55] |
| Acetaminophen, antipyrine, bisphenol A, caffeine, metoprolol, testosterone. Concentratoin: 1.5 and 2.9 mg L−1 (10−5 M each). Processes: Sun, O3, Fe(III), or P25 TiO2 combinations. Also, H2O2 for photo-Fenton. | Fe(III) (homog.: 2.79 mg L−1, pH 3), TiO2 (heterog.: 200 mg L−1, pH 7). In some cases: Fe(III)/H2O2 = 6.09 mass ratio. 13 mg L−1 ozone in gas. | The Sun. Average solar radiation: 40 W m−2. | Four borosilicate glass tube CPCs (29.4 × 75 cm). Collector surface 0.25 m2. Illumination volume: 1.8 L. Tilted 45° to the south. Parabolic anodized aluminum reflectors. Turbulent regime. Semi-batch mode. | Concentrations and TOC removal. Energy and ozone demands. Kinetic regimes. Kinetics. | [56] |
| Acetaminophen, antipyrine, bisphenol A, caffeine, metoprolol, testosterone in secondary WWSE effluent (BOD = 10, COD = 58.6; TOC = 20 mg L−1). Presence of anionic ions. Concentrations between 1.5 and 0.2 mg L−1. Processes: Sun, O3, Fe(III), or P25 TiO2 and their combinations. Also, H2O2 in some cases. | Fe(III) (homog.: 2.8 mg L−1, pH 3), TiO2 (heterog.: 200 mg L−1, pH 7). 13 mg L−1 ozone in gas. | The Sun. Average solar radiation: 40 Wm−2. | Four borosilicate glass tube CPCs (29.4 × 75 cm). Collector surface 0.25 m2. Illumination volume: 1.8 L. Tilted 45° to the south. Parabolic anodized aluminum reflectors. Turbulent regime. Semi-batch mode. Reaction time: 5 h (+30 min, previous for adsorption). | Concentrations and TOC removal. Also, ions and total phenol concentration. Daphnia magna ecotoxicity measurements. Measurements of HO· radicals concentration with p-chlorobenzoic acid as a probe compound. Biodegradability as BOD/COD. Economic considerations. | [57] |
| Diuron, o-phenylphenol, 2-methyl-4-chlorophenoxyaceticacid (MCPA), and tert-buthylazine (5 mg L−1 each). Processes: O3, UVA-Vis, O3/cat, O3/UVA-Vis, O2/cat/UVA-Vis, O3/cat/UVA-Vis. | TiO2 and 0.5–0.8 wt. % B-TiO2 0.33 g L−1. Ozone in the inlet gas: 5 mg L−1 and 10 L h−1. | 1000 W Xe lamp. Incident radiation flux: 8.96 × 10−4 Einstein min−1. Radiation intensity: 500 W m−2. | Glass-made agitated tank provided with gas inlet, gas outlet, and liquid sampling ports in a solar simulator box. | Synthesis and characterization: ICP-OES, N2 adsorption–desorption, XRD, XPS, and DRUV-Vis spectroscopy (3.01 and 3.03 eV band gap) Compound concentration. TOC, dissolved ozone, and H2O2 concentrations. B leached. | [58] |
| Acetaminophen (ACM), antipyrine (ANT), caffeine (CAF), ketorolac (KET), metoprolol, sulfamethoxazole (SFX), carbamazepine (CARB), hydrochlorothiazide (HCT), and diclofenac (DIC). In WWPE doped: 200 μg L−1 each. Processes: solar photocatalysis with O3/TiO2, solar photo-Fenton, or ozonation. | Three catalysts: 1. pH 3 with 2.8 mg L−1 Fe(III). 2. 150 mg L−1Fe3O4. 3. Natural pH with 250 mg L−1 P25 TiO2. Ozone in the inlet gas: 33.6 L h−1 and 13 mg L−1. | Radiation source: Sun. | Aerobic tank: HRT of 7 h, biomass sludge aged 5–6 days. MLVSS.MLSS−1: 0.8. Oxygen: 2–4 mg L−1. Reactor Volume: 5 L (1.8 L of irradiated volume) compound. Parabolic collector. Four borosilicate glass tube CPCs (29.4 × 75 cm). | Aerobic degradation followed by AOP, solar photocatalysis. ECs concentration, TOC, COD, ecotoxicty. Accumulated UV-vis energy calculated. | [59] |
| Bisphenol A and oxalic acid (OA), 10 mg L−1 at 1 mL min−1 and 500 mgh−1. | Graphitic carbon nitride: g-C3N4 composed of numerous interconnected nanosheets. Concentration: 0.5 g L−1 | High-pressure Xe long-arc lamp, jacketed by a quartz thimble (GXZ500 W). Filter with Na2NO2 to cut λ < 400 nm. | 1 L glass tubular photoreactor (8.5 × 40 cm). | Synthesis (from urea) and characterization (TEM, FTIR, BET (67 m2/g), XRD, UV-Vis (2.7 eV, 450 nm max.). Rates for oxalic acid and BPA higher than sum of single processes: O3 and UV-Vis/cat/O2. Tert-butanol, (TBA), triethanolamine (TEOA), and benzoquinone (BQ) as HO·, hole and O2—scavengers. | [60] |
| Oxalic acid, 0.11 mM. | gC3N4-reduced graphene oxide (rGOxide) 0.2 g/L. Ozone: 75 mgh−1. | As in Reference [56]. | As in Reference [56]. | Catalyst characterization. 2% rGO leads to best results for OA removal. Catalyst activity and stability. Basic mechanism. | [61] |
| Phenol in water and urban wastewater: 50 mg/L (DOC: 20 mg L−1, COD: 40 mg L−1, pH 7.4) Processes: Ozonation, photocatalysis, and photocatalytic ozonation | Ag, Cu, Fe on TiO2: 0.5 g L−1 Ozone: 20.83 mg L−1 min−1 | Heraeus TQ 150 W immersion medium-pressure Hg lamp 70 mW cm2. The lamp emission spectrum has main peaks at 253.7, 313, and 366 nm in the UV range and 436, 546, and 578 nm in the visible range. For solar runs: 37.6 mW cm−2. | For UV runs: cylindrical quartz photochemical reactor (0.7 L) wrapped in aluminum foil. For solar runs: glass tubular reactor (1 m in length and 0.04 m in diameter) and a parabolic solar collector. | Best catalyst: Fe-TiO2 which presents the highest BET area and higher λ visible absorption (530 nm). Phenol concentration. COD, Langmuir kinetics applied simplified to pseudo first order kinetics. Synergic index, pseudo first rate constants calculated. | [62] |
| t-Butilazina: 5 mg L−1. Processes: Adsorption onto AC and multi-walled carbon nanotube (MWCNT), UV photolysis, UV/H2O2, single ozonation, O3/H2O2, catalytic ozonation (AC, MWCNT and TiO2 as catalysts) and some solar driven processes such as photo ozonation, TiO2-photocatalytic oxidation, and TiO2-photocatalytic ozonation. | AC (DARCO®, 12–20 mesh) and MWCNTs (purity > 95%) carbon, P25 TiO2, and other TiO2 prepared and TiO2-MWCNT. Ozone: 10 mg L−1, 20 L h−1. | Low-pressure Hg lamp with emission at 254 nm (Heraeus, model TNN15/32). Average fluence rate: 0.6 W–1 (2.9 mW cm−2). For UVA-visible: 1000 W Xe lamp (300–800 nm). 581 W m−2 (62 Wm−2 UV-A irradiance. | For UVC photolysis and UV/H2O2: photoreactor provided with a central quartz well. For O3 and O3/cat runs: 400 mL semi-batch spherical Pyrex-made reactor provided with magnetic agitation. For photoprocesses: The same reactor as in ozone processes inside a Suntest solar simulator equipment | Characterization: XRD, SBET, TEM, FTIR. Isotherm and different AOPs and adsorption kinetics TBA concentrations and intermediates by HPLC-qTOF. | [63] |
| N,N-diethyl-meta-toluamide (DEET): 5 mg L−1. Processes: Different AOPs and adsorption. | Two CeO2 catalysts: nanorod and nanocubes. Hydrothermal method. Concentration: 0.25 g L−1. Ozone: 10 mg L−1. 15 L h−1. | 1500 W Xe lamp: 550 W m−2 and 300 to 800 nm or 400 to 800 nm with filter. | Semi-batch borosilicate glass-made round flask in a Suntest CPS solar simulator. | Catalyst preparation and characterization: XRD, XPS, SBET, DR UV-Vis. DEET concentration, TOC, O3 dis. H2O2, short chain carboxylic acids. Pseudo first order kinetics. | [64] |
| N,N-diethyl-meta-toluamide (DEET): 15 mg L−1. Ozone processes: 10 mg L−1, 15 L h−1 gas flow rate. | Monoclinic WO3 calcined at 600 °C (see Mena et al., 2015): 0.25 g L−1. | 1500 W air-cooled Xe arc lamp with emission restricted to visible light (λ > 390 nm) because of quartz, glass, and polyester cut-off filters. 550 W m−2. | 0.5 L glass-made spherical reactor in the chamber of a box simulator. | HPLC-qTOF identification of intermediates. Mechanism and kinetics based on TOC removal. Scavengers used: t-butanol and oxalate. Arrhenius equation determined for DEET-O3 reaction. | [65] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán, F.J.; Rey, A. Solar or UVA-Visible Photocatalytic Ozonation of Water Contaminants. Molecules 2017, 22, 1177. https://doi.org/10.3390/molecules22071177
Beltrán FJ, Rey A. Solar or UVA-Visible Photocatalytic Ozonation of Water Contaminants. Molecules. 2017; 22(7):1177. https://doi.org/10.3390/molecules22071177
Chicago/Turabian StyleBeltrán, Fernando J., and Ana Rey. 2017. "Solar or UVA-Visible Photocatalytic Ozonation of Water Contaminants" Molecules 22, no. 7: 1177. https://doi.org/10.3390/molecules22071177





