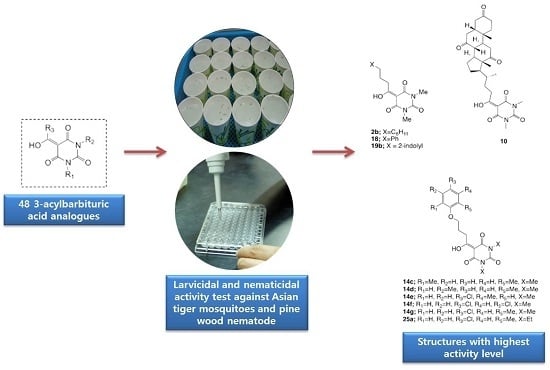
Larvicidal and Nematicidal Activities of 3-Acylbarbituric Acid Analogues against Asian Tiger Mosquito, Aedes albopictus, and Pine Wood Nematode, Bursaphelenchus xylophilus
Abstract
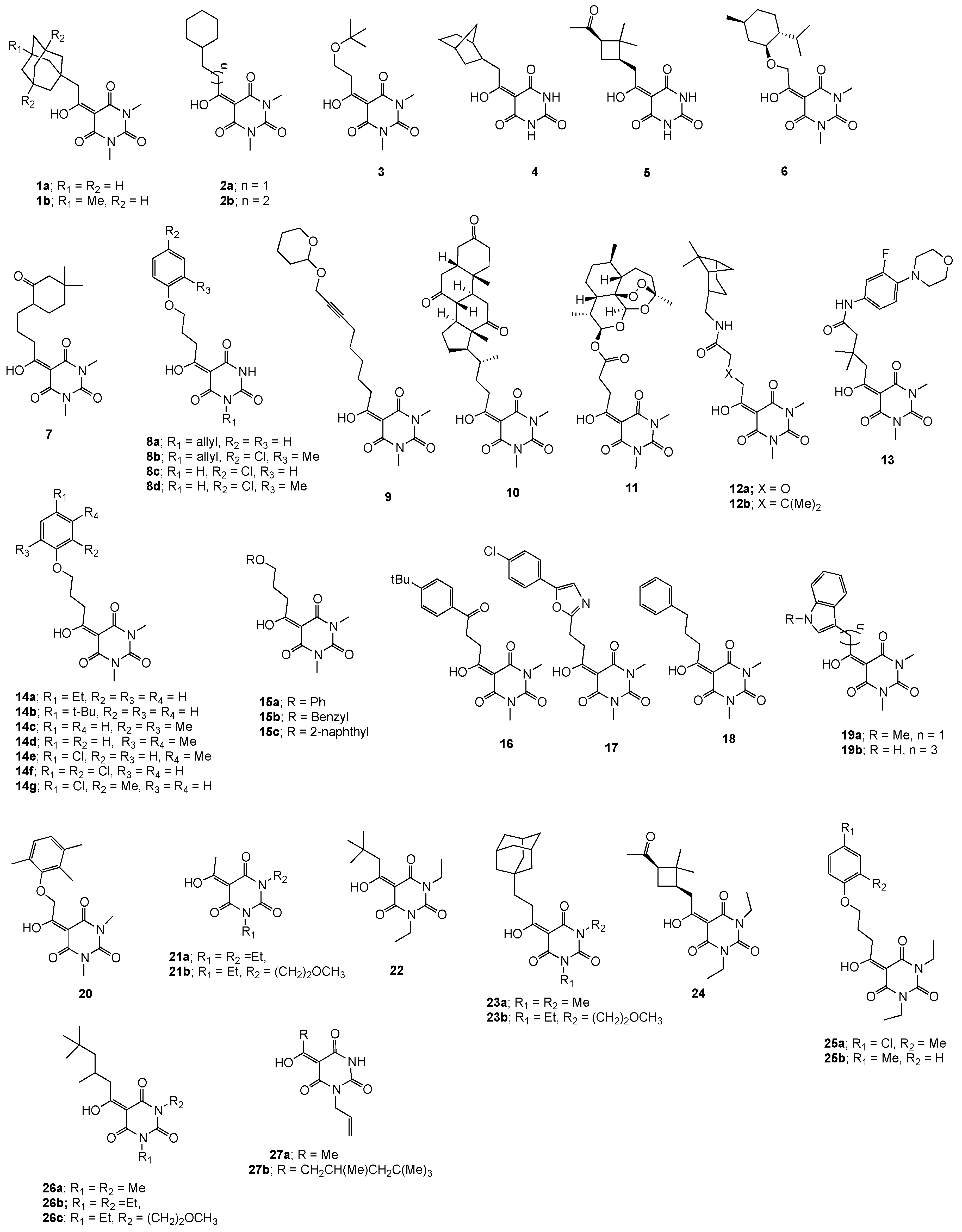
:1. Introduction
2. Results and Discussion
2.1. Larvicidal and Nematicidal Activities of Barbituric Acids
2.2. Physicochemical Property–Larvicidal Activity Relationships
3. Materials and Methods
3.1. Chemicals
3.2. Insects
3.3.Collection of the Pine Wood Nematode
3.4. Larvicidal Activity Test
3.5. Nematicidal Activity Test
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonizzoni, M.; Gasperi, G.; Chen, X.; James, A.A. The invasive mosquito species Aedes albopictus: Current knowledge and future perspectives. Trends Parasitol. 2013, 29, 460–468. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Insecticides Use for Vector-Borne Disease Control, a 10 year Assessment (2000–2009), 5th ed.; World Health Organization: Geneva, Switzerland, 2011; pp. 7–26. [Google Scholar]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; della Torre, A.; Ranson, H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aetypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidence, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Abban, E.K.; Samman, J. Preliminary observations on the effect of the insect larvicide abate on fish catches in the river Oti, Ghana. Environ. Pollut. A 1980, 21, 307–311. [Google Scholar] [CrossRef]
- Anadu, D.I.; Anaso, H.U.; Onyeka, O.N.D. Acute toxicity of the insect lavicide Abate® on the fish Tilapia melanopleura and the dragonfly larvae Neurocordelia virginiensis. J. Environ. Sci. Health Part B 1996, 31, 1363–1375. [Google Scholar] [CrossRef]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. Pine Wilt Disease; Springer: Tokyo, Japan, 2008; pp. 2–40. ISBN 9784431756545. [Google Scholar]
- Lee, S.M.; Chung, Y.J.; Moon, Y.S.; Lee, S.G.; Lee, D.W.; Choo, H.Y.; Lee, C.K. Insecticidal activity and fumigation conditions of several insecticides against Japanese pine sawyer (Monochamus alternatus) larvae. J. Korean For. Soc. 2003, 92, 191–198. [Google Scholar]
- Korea Forest Research Institute. Development of Control Method for Pine Wilt Disease; Korea Forest Research Institute: Seoul, Korea, 2013; pp. 103–149. ISBN 1114003770032801. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seo, S.M.; Lee, S.G.; Shin, S.C.; Park, I.K. Nematicidal activity of plant essential oils and components from coriander (Coriandrum sativum), oriental sweetgum (Liquidambar orientalis), and valerian (Valeriana wallichii) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2008, 56, 7316–7320. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Kim, J.; Kim, E.; Park, H.M.; Kim, Y.J.; Park, I.K. Structure-activity relationship of aliphatic compounds for nematicidal activity against pine wood nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2010, 58, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Park, I.K. Larvicidal activity of Amyris balsamifera, Daucus carota and Pogostemon cablin essential oils and their components against Culex pipiens pallens. J. Asia Pac. Entomol. 2012, 15, 631–634. [Google Scholar] [CrossRef]
- Seo, S.M.; Kim, J.; Koh, S.H.; Ahn, Y.J.; Park, I.K. Nematicidal activity of natural ester compounds and their analogues against pine wood nematode, Bursaphelenchus xylophilus. J. Agric. Food Chem. 2014, 62, 9103–9108. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Jung, C.S.; Kang, J.; Lee, H.R.; Kim, S.W.; Hyun, J.; Park, I.K. Larvicidal and acetylcholinesterase inhibitory activities of Apiaceae plant essential oils and their constituents against Aedes albopictus and formulation development. J. Agric. Food Chem. 2015, 63, 9977–9986. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.C.; Moloney, M.G. Antibacterial barbituric acid analogues inspired from natural 3-acyltetramic acids: synthesis, tautomerism and structure and physicochemical property-antibacterial activity relationships. Molecules 2015, 20, 3582–3627. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.N.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Thermal solvent-free synthesis of novel pyrazolyl chalcones and pyrazolines as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 2860–2865. [Google Scholar] [CrossRef] [PubMed]
- Faidallah, H.M.; Khan, K.A. Synthesis and biological evaluation of new barbituric acid and thiobarbituric acid fluoro analogs of benzenesulfonamides as antidiabetic and antibacterial agents. J. Fluor. Chem. 2012, 142, 96–104. [Google Scholar] [CrossRef]
- Kay, I.T.; Peacock, F.C.; Waring, W.S. 5-Acyl Barbituric Acid Derivatives. U.S. Patent 3,828,043 A, 6 August 1974. [Google Scholar]
- Hirono, Y.; Ishikawa, H.; Iwataki, I.; Sawaki, M.; Nomura, O. Barbituric Acid Derivatives. U.S. Patent 3,999,974 A, 28 December 1976. [Google Scholar]
- Perumalsamy, H.; Jang, M.J.; Kim, J.R.; Kadarkarai, M.; Ahn, Y.J. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasite Vector 2015, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. Emamectin benzoate as a candidate for trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2000, 56, 937–941. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, L.Y.; Lai, D.; Xu, H.H. The nematicidal and proteomic effects of Huanong AVM (analog of avermectin) on the pine-wilt nematode, Bursaphelenchus xylophilus. Pestic. Biochem. Physiol. 2010, 98, 224–230. [Google Scholar] [CrossRef]
- Park, I.K.; Lee, S.G.; Shin, S.C.; Park, J.D.; Ahn, Y.J. Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species. J. Agric. Food Chem. 2002, 50, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS/STAT User’s Guide; Version 9.1.3; SAS Institute: Cary, NC, USA, 2004. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compounds | Mortality of Ae. albopictus (%, Mean ± SE) | Mortality of B. xylophilus (%, Mean ± SE) | ||||
|---|---|---|---|---|---|---|
| Concentration 1 | Concentration | |||||
| 10 | 5 | 2.5 | 1.25 | 10 | 5 | |
| 1a | 30.0 ± 8.1bcdef 2 | - 3 | - | - | 0.2 ± 0.2b | - |
| 1b | 2.5 ± 5.0f | - | - | - | 1.2 ± 0.4b | - |
| 2a | 30.0 ± 14.1bcdef | - | - | - | 1.2 ± 0.5b | - |
| 2b | 75.0 ± 20.8ab | 0c | - | - | 0.9 ± 0.4b | - |
| 3 | 0f | - | - | - | 0.2 ± 0.2b | - |
| 4 | 2.5 ± 5.0f | - | - | - | 0.3 ± 0.3b | - |
| 5 | 0f | - | - | - | 0.5 ± 0.3b | - |
| 6 | 0f | - | - | - | 0.7 ± 0.2b | - |
| 7 | 0f | - | - | - | 0.6 ± 0.3b | - |
| 8a | 0f | - | - | - | 0.2 ± 0.2b | - |
| 8b | 10def | - | - | - | 0.2 ± 0.2b | - |
| 8c | 0f | - | - | - | 0.5 ± 0.5b | - |
| 8d | 0f | - | - | - | 0.3 ± 0.3b | - |
| 9 | 0f | - | - | - | 3.4 ± 1.6b | - |
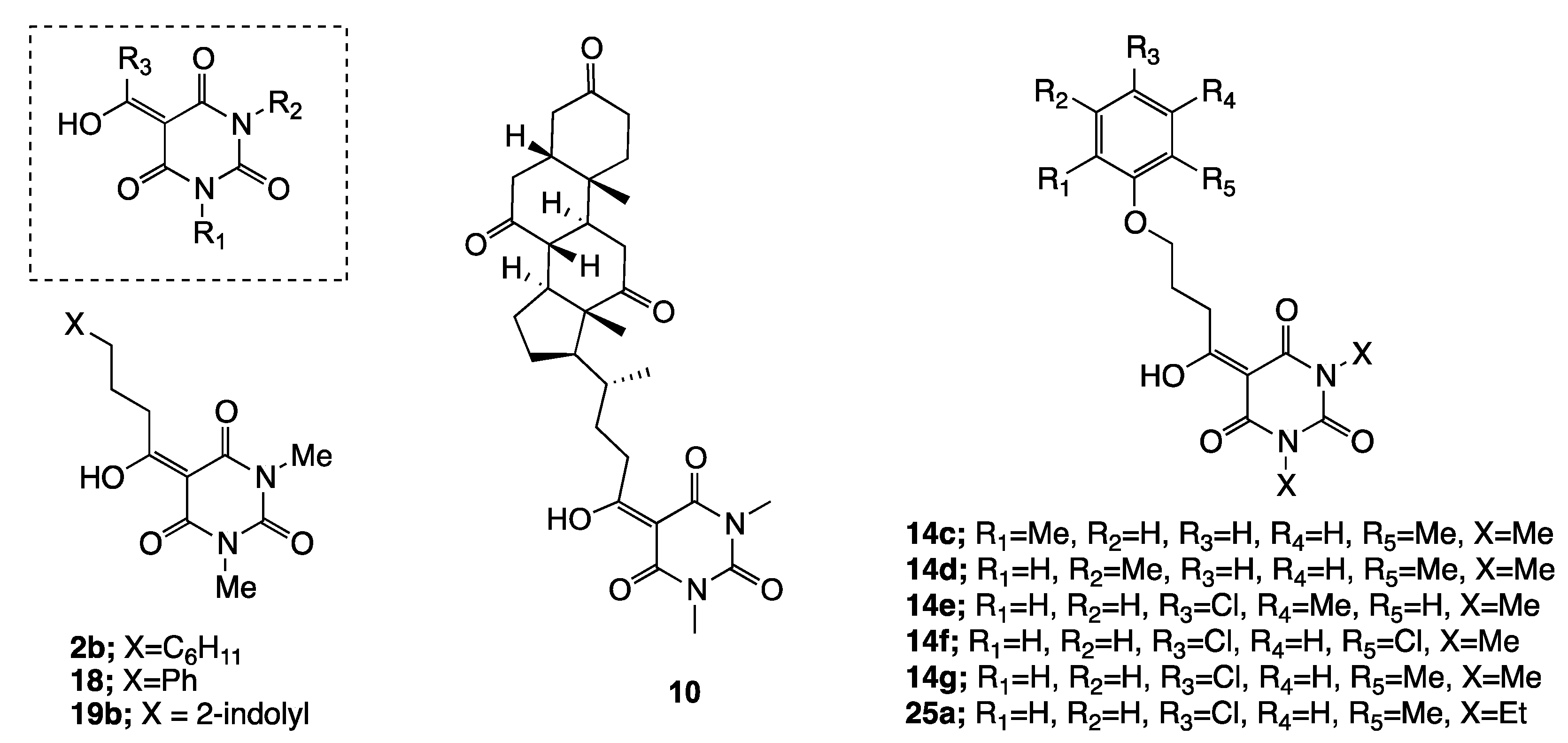
| 10 | 100a 2 | 100a | 100a | 100a | 0.5 ± 0.3b | - |
| 11 | 5.0 ± 5.77f | - | - | - | 0.5 ± 0.3b | - |
| 12a | 0f | - | - | - | 0.5 ± 0.5b | - |
| 12b | 0f | - | - | - | 0.9 ± 0.4b | - |
| 13 | 2.5 ± 5.0f | - | - | - | 0b | - |
| 14a | 60.0 ± 14.1abcde | 2.5 ± 5.00bc | - | - | 0.3 ± 0.3b | - |
| 14b | 12.5 ± 9.5def | - | - | - | 1.4 ± 0.5b | - |
| 14c | 77.5 ± 15.0ab | 37.5 ± 20.62bc | 35.0 ± 17.32b | - | 0b | - |
| 14d | 97.5 ± 5.0a | 37.5 ± 29.36bc | - | - | 0.8 ± 0.6b | - |
| 14e | 77.5 ± 18.9ab | 15.0 ± 5.77bc | - | - | 1.0 ± 0.4b | - |
| 14f | 87.5 ± 12.5a | 40.0 ± 32.66bc | 22.5 ± 15.00bc | - | 0.8 ± 0.5b | - |
| 14g | 100a | 100a | 90a | 2.5 ± 5.00b | 0.2 ± 0.2b | - |
| 15a | 7.5 ± 9.5ef | - | - | - | 1.6 ± 0.2b | - |
| 15b | 0f | - | - | - | 1.3 ± 0.6b | - |
| 15c | 75.0 ± 20.8ab | 55.0 ± 23.80ab | 10c | - | 0.3 ± 0.3b | - |
| 16 | 0f | - | - | - | 0.9 ± 0.5b | - |
| 17 | 30.0 ± 16.3bcdef | - | - | - | 0.9 ± 0.4b | - |
| 18 | 50.0 ± 8.1abcdef | - | - | - | 93.4 ± 2.3a | 3.5 ± 0.9 |
| 19a | 10 ± 11.5def | - | - | - | 1.9 ± 1.1b | - |
| 19b | 92.5. ± 9.5a | 10.0 ± 8.16bc | - | - | 0.6 ± 0.6b | - |
| 20 | 5.0 ± 5.7f | - | - | - | 0.3 ± 0.3b | - |
| 21a | 77.5 ± 18.9ab | 0c | - | - | 0.5 ± 0.5b | - |
| 21b | 0f | - | - | - | 0.9 ± 0.6b | - |
| 22 | 7.5 ± 9.57cdef | - | - | - | 1.3 ± 0.5b | - |
| 23a | 0f | - | - | - | 0.4 ± 0.3b | - |
| 23b | 0f | - | - | - | 1.2 ± 1.2b | - |
| 24 | 0f | - | - | - | 0.6 ± 0.6b | - |
| 25a | 70.0 ± 18.26abc | 10.0 ± 11.55bc | - | - | 0.5 ± 0.3b | - |
| 25b | 27.5 ± 15.00bcdef | - | - | - | 1.7 ± 1.1b | - |
| 26a | 0f | - | - | - | 0b | - |
| 26b | 62.5 ± 9.57abcd | 0c | - | - | 0.5 ± 0.3b | - |
| 26c | 17.5 ± 9.57cdef | - | - | - | 0.0 ± 0.0b | - |
| 27a | 0f | - | - | - | 0.0 ± 0.0b | - |
| 27b | 5.0 ± 5.7f | - | - | - | 0.5 ± 0.3b | - |
| Control | 0f | 0c | 0c | 0b | 0b | 0 |
| F48,147 = 57.92, p < 0.0001 | F13,42 = 21.49, p < 0.0001 | F5,18 = 81.19, p < 0.0001 | F2,9 = 1561.0, p < 0.0001 | F48,147 = 477.83, p < 0.0001 | ||
| Compound | LC50 (μg/mL) | Regression Line | 95% CI 1 | χ2 |
|---|---|---|---|---|
| 10 | 0.22 | y = 1.895χ + 6.253 | 0.17–0.29 | 0.547 |
| Temephos | 0.0093 | y = 2.844χ + 10.850 | 0.0071–0.0118 | 3.368 |
| Compounds | MW 1 | MSA 2 | PSA 3 | %PSA 4 | ClogP 5 | ClogD7.4 6 | H-D 7/H-A 8 | RB 9 |
|---|---|---|---|---|---|---|---|---|
| 1a | 332 | 470 | 77.9 | 16.6 | 1.02 | 0.00 | 1/4 | 2 |
| 1b | 346 | 506 | 77.9 | 15.4 | 1.45 | 0.46 | 1/4 | 2 |
| 2a | 294 | 441 | 77.9 | 17.7 | 1.00 | 0.10 | 1/4 | 3 |
| 2b | 308 | 471 | 77.9 | 16.5 | 1.40 | 0.54 | 1/4 | 4 |
| 3 | 284 | 432 | 87.2 | 20.2 | −0.90 | −2.26 | 1/5 | 4 |
| 4 | 264 | 343 | 95.5 | 27.8 | −0.13 | −1.34 | 3/4 | 2 |
| 5 | 294 | 406 | 113 | 27.8 | −0.04 | −1.28 | 3/5 | 3 |
| 6 | 352 | 545 | 87.2 | 16.0 | 1.26 | −1.35 | 1/5 | 4 |
| 7 | 350 | 533 | 95.0 | 17.8 | 1.54 | 0.61 | 1/5 | 4 |
| 8a | 330 | 441 | 95.9 | 21.7 | 1.00 | −0.21 | 2/5 | 7 |
| 8b | 379 | 489 | 95.9 | 19.6 | 1.98 | 0.48 | 2/5 | 7 |
| 8c | 325 | 392 | 105 | 26.8 | 0.53 | −1.24 | 3/5 | 5 |
| 8d | 339 | 424 | 105 | 24.8 | 0.99 | −0.63 | 3/5 | 5 |
| 9 | 392 | 584 | 96.4 | 16.5 | 1.05 | −0.06 | 1/6 | 9 |
| 10 | 541 | 803 | 129 | 16.1 | 3.19 | 2.10 | 1/7 | 4 |
| 11 | 523 | - 10 | 141 | - 9 | 1.71 | 0.17 | 1/9 | 5 |
| 12a | 407 | 599 | 116 | 19.4 | −0.44 | −3.42 | 2/6 | 6 |
| 12b | 434 | 678 | 107 | 15.8 | 1.32 | 0.15 | 2/5 | 6 |
| 13 | 476 | 673 | 119 | 17.7 | 1.12 | −0.34 | 2/7 | 6 |
| 14a | 346 | 503 | 87.2 | 17.3 | 1.36 | 0.15 | 1/5 | 6 |
| 14b | 374 | 569 | 87.2 | 15.3 | 2.13 | 0.91 | 1/5 | 6 |
| 14c | 346 | 504 | 87.2 | 17.3 | 1.44 | 0.41 | 1/5 | 5 |
| 14d | 346 | 505 | 87.2 | 17.3 | 1.44 | 0.40 | 1/5 | 5 |
| 14e | 367 | 488 | 87.2 | 17.9 | 1.49 | 0.03 | 1/5 | 5 |
| 14f | 387 | 473 | 87.2 | 18.4 | 1.54 | −0.32 | 1/5 | 5 |
| 14g | 367 | 489 | 87.2 | 17.8 | 1.49 | 0.03 | 1/5 | 5 |
| 15a | 318 | 440 | 87.2 | 19.8 | 0.50 | −0.65 | 1/5 | 5 |
| 15b | 332 | 471 | 87.2 | 18.5 | 0.50 | −0.59 | 1/5 | 6 |
| 15c | 368 | 504 | 87.2 | 17.3 | 1.50 | 0.37 | 1/5 | 5 |
| 16 | 372 | 549 | 95.0 | 17.3 | 1.51 | 0.05 | 1/5 | 5 |
| 17 | 390 | 483 | 104 | 21.5 | 0.70 | −0.82 | 1/5 | 4 |
| 18 | 302 | 424 | 77.9 | 18.4 | 1.20 | 0.27 | 1/4 | 4 |
| 19a | 327 | 437 | 82.9 | 19.0 | 0.76 | −0.63 | 1/4 | 2 |
| 19b | 341 | 464 | 93.7 | 20.2 | 1.30 | 0.42 | 2/4 | 4 |
| 20 | 332 | 475 | 87.2 | 18.4 | 1.40 | −1.46 | 1/5 | 3 |
| 21a | 226 | 320 | 77.9 | 24.3 | −0.59 | −1.44 | 1/4 | 2 |
| 21b | 256 | 367 | 87.2 | 23.8 | −1.10 | −1.99 | 1/5 | 4 |
| 22 | 282 | 447 | 77.9 | 17.4 | 1.04 | 0.11 | 1/4 | 4 |
| 23a | 346 | 501 | 77.9 | 15.5 | 1.42 | 0.51 | 1/4 | 3 |
| 23b | 404 | 613 | 87.2 | 14.2 | 1.60 | 0.70 | 1/5 | 7 |
| 24 | 350 | 536 | 95.0 | 17.7 | 1.14 | 0.15 | 1/5 | 5 |
| 25a | 395 | 553 | 87.2 | 15.8 | 2.17 | 0.80 | 1/5 | 7 |
| 25b | 360 | 537 | 87.2 | 16.2 | 1.65 | 0.53 | 1/5 | 7 |
| 26a | 296 | 474 | 77.9 | 16.4 | 1.48 | 0.62 | 1/4 | 4 |
| 26b | 324 | 538 | 77.9 | 14.5 | 2.16 | 1.36 | 1/4 | 6 |
| 26c | 354 | 585 | 87.2 | 14.9 | 1.65 | 0.81 | 1/5 | 8 |
| 27a | 210 | 256 | 86.7 | 33.9 | −0.78 | −1.80 | 2/4 | 2 |
| 27b | 308 | 473 | 86.7 | 18.3 | 1.97 | 1.05 | 2/4 | 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.-M.; Lee, H.-R.; Lee, J.-E.; Jeong, Y.-C.; Kwon, H.-W.; Moon, J.-K.; Moloney, M.G.; Park, I.-K. Larvicidal and Nematicidal Activities of 3-Acylbarbituric Acid Analogues against Asian Tiger Mosquito, Aedes albopictus, and Pine Wood Nematode, Bursaphelenchus xylophilus. Molecules 2017, 22, 1196. https://doi.org/10.3390/molecules22071196
Seo S-M, Lee H-R, Lee J-E, Jeong Y-C, Kwon H-W, Moon J-K, Moloney MG, Park I-K. Larvicidal and Nematicidal Activities of 3-Acylbarbituric Acid Analogues against Asian Tiger Mosquito, Aedes albopictus, and Pine Wood Nematode, Bursaphelenchus xylophilus. Molecules. 2017; 22(7):1196. https://doi.org/10.3390/molecules22071196
Chicago/Turabian StyleSeo, Seon-Mi, Hyo-Rim Lee, Ji-Eun Lee, Yong-Chul Jeong, Hyung-Wook Kwon, Joon-Kwan Moon, Mark G. Moloney, and Il-Kwon Park. 2017. "Larvicidal and Nematicidal Activities of 3-Acylbarbituric Acid Analogues against Asian Tiger Mosquito, Aedes albopictus, and Pine Wood Nematode, Bursaphelenchus xylophilus" Molecules 22, no. 7: 1196. https://doi.org/10.3390/molecules22071196