Synergistic Promotion on Tyrosinase Inhibition by Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of Antioxidant Solutions
2.3. DPPH Free Radical-Scavenging Capacity Assay
2.4. Preparation of Individual Solutions and Mixtures
2.5. Mushroom Tyrosinase Inhibitory Assay
2.6. Molecular Docking Strategy
2.7. Drug Synergism Analysis of RSE and OXYR in PIG1 Cell
2.7.1. Cell Culture
2.7.2. UVB Irradiation Treatment
2.7.3. Detection of Cell Proliferation Activity by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium Bromide (MTT) Assay
2.7.4. Tyrosinase Activity Assay
2.8. Statistical Analysis
3. Results and Discussion
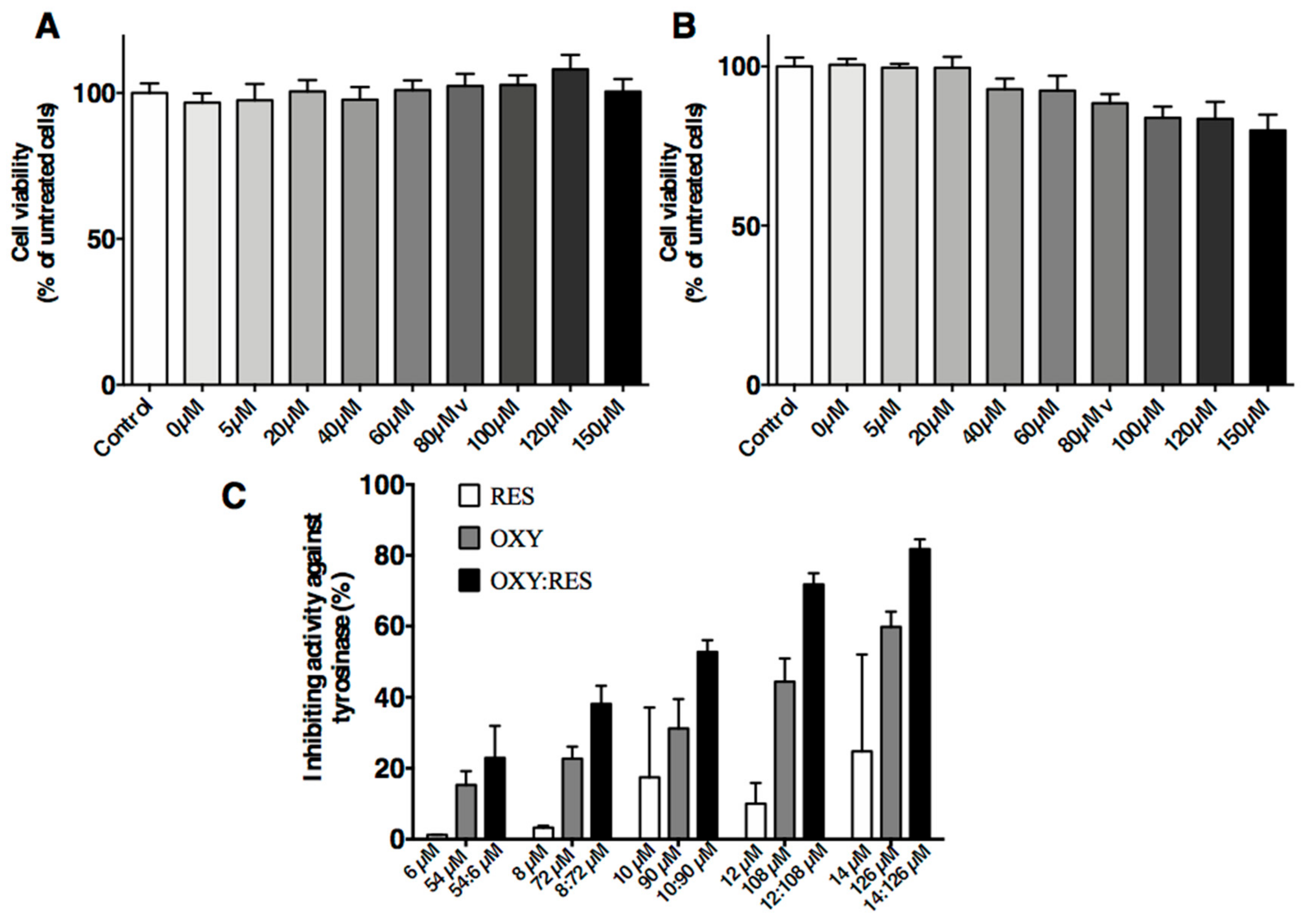
3.1. The IC50 Values of Antioxidant Solutions
3.2. The IC50 Values of Individual Solutions of Tyrosinase Inhibitory Activities
3.3. Tyrosinase Inhibition and Synergistic Effects of Antioxidant and Tyrosinase Inhibitor
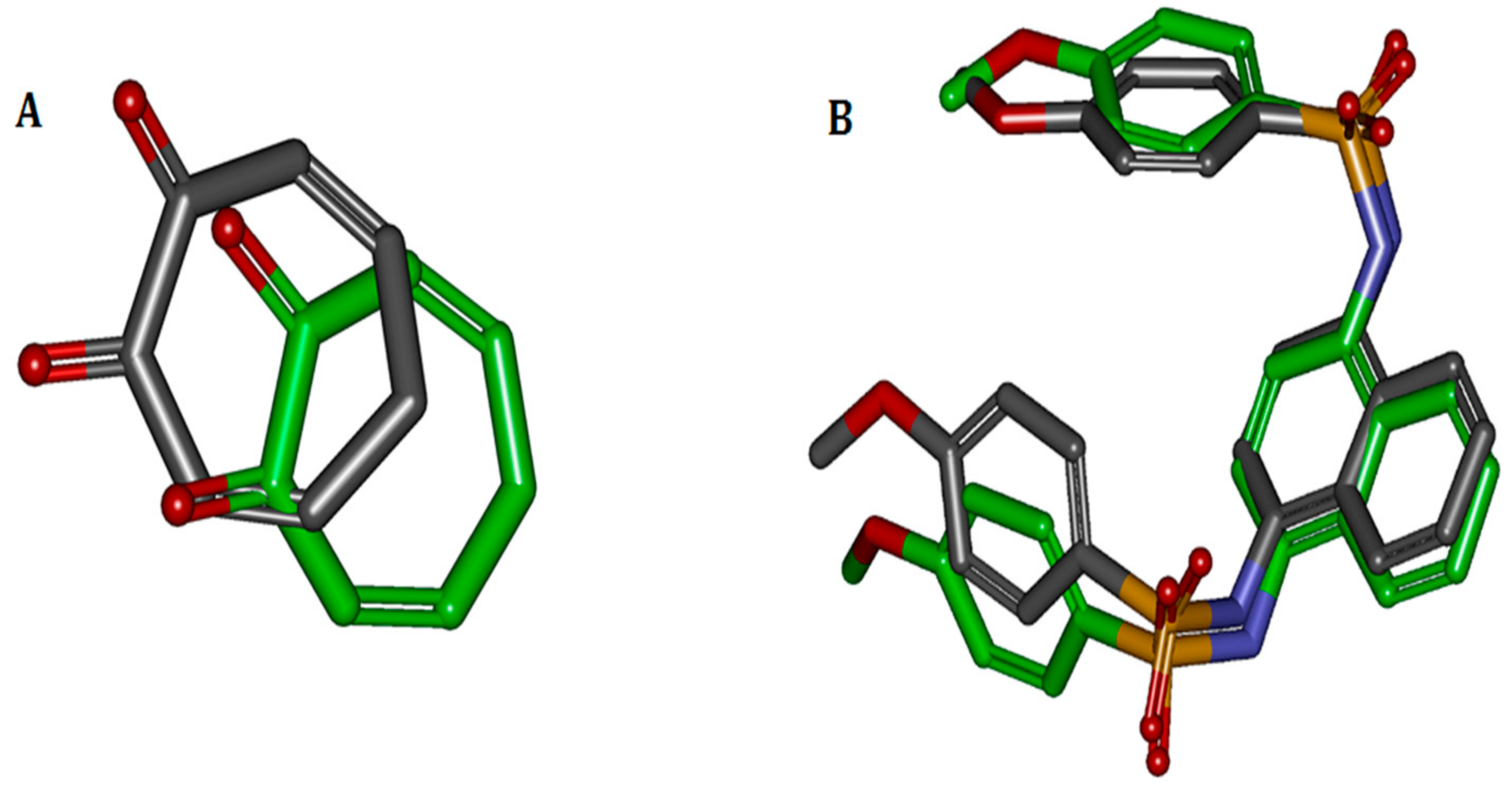
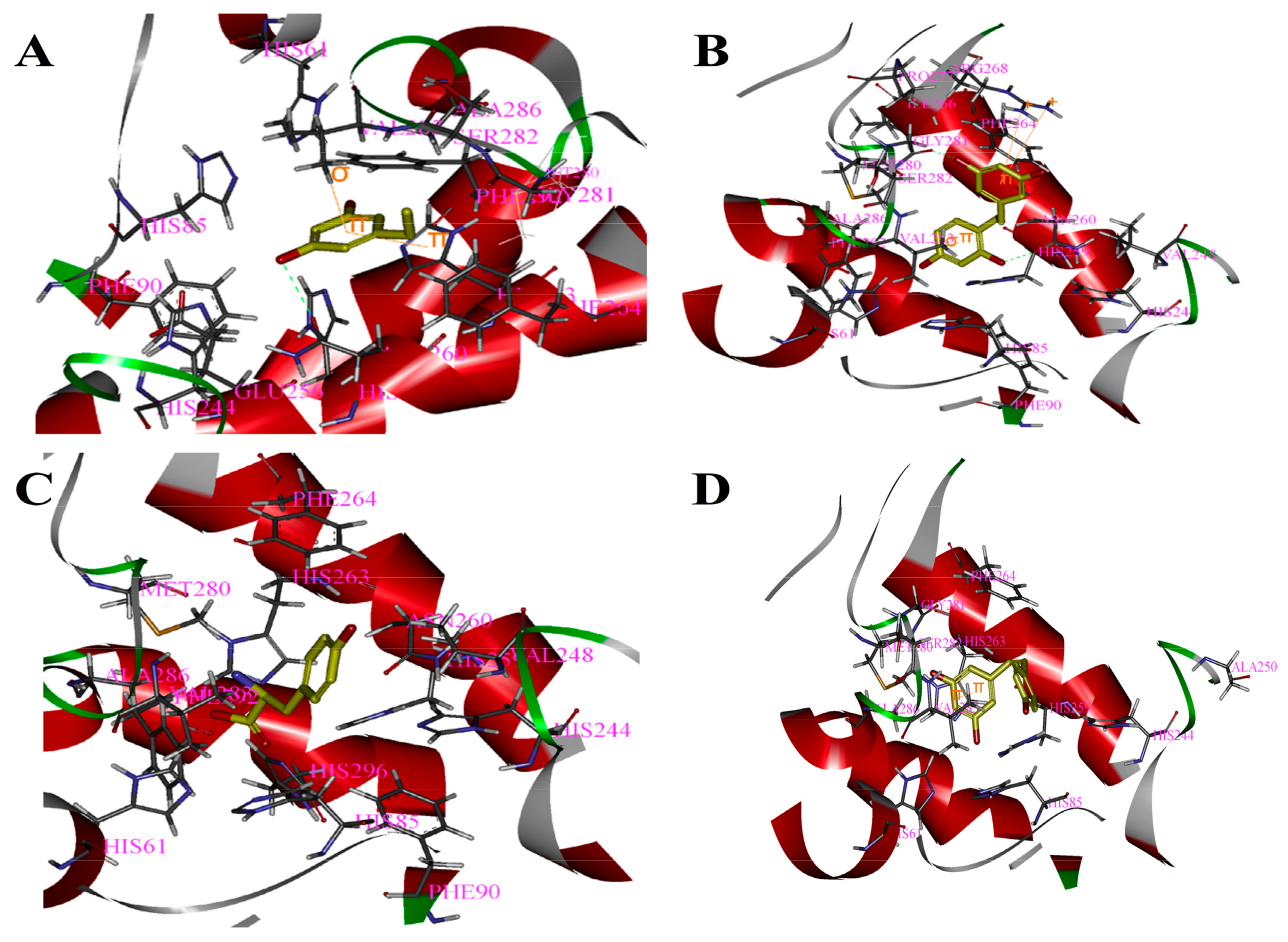
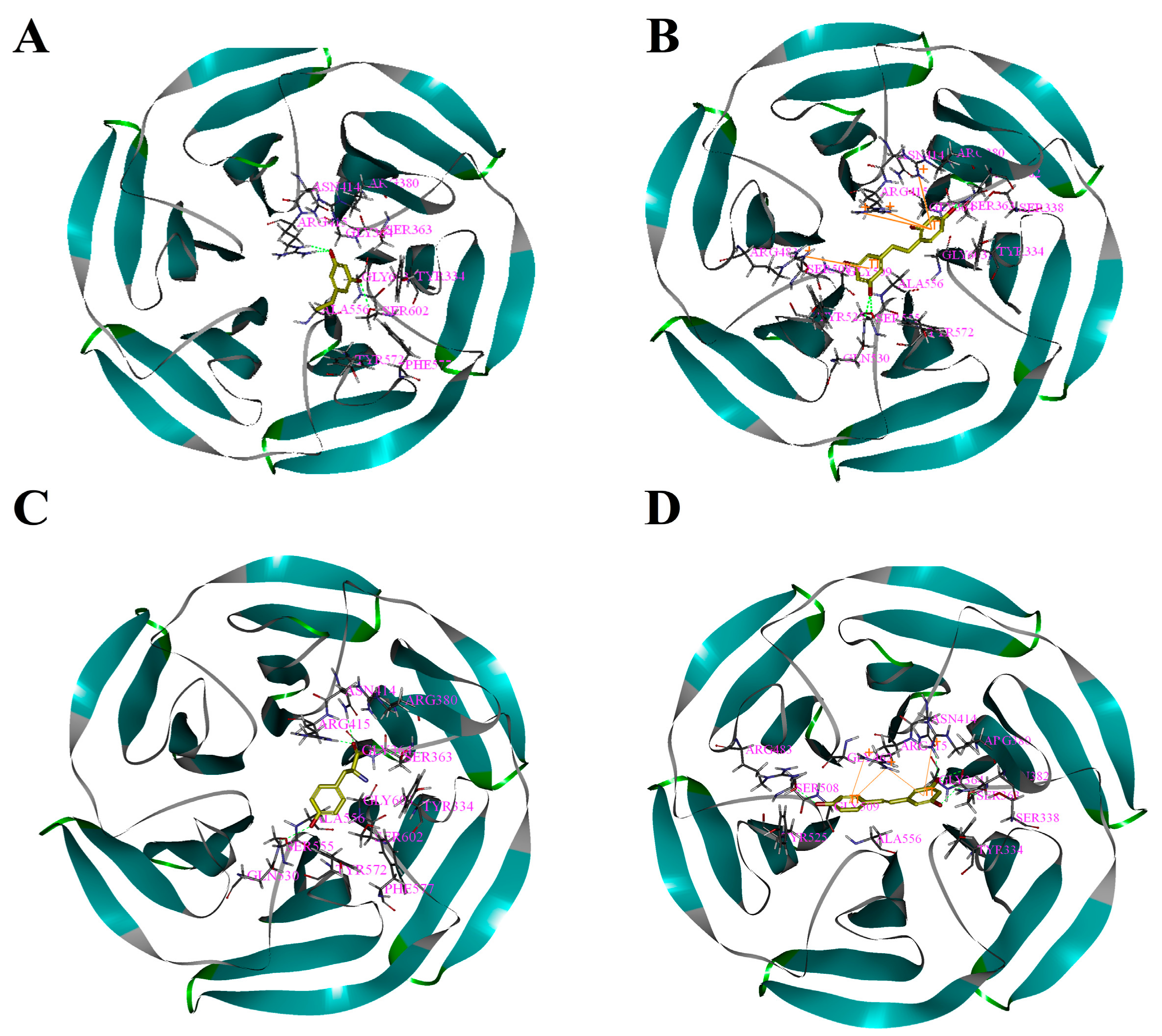
3.4. Molecular Docking Strategy
3.5. Drug Synergism Analysis of RSE and OXYR in PIG1 Cell
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birch-Machin, M.A.; Bowman, A. Oxidative stress and ageing. Br. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Lim, S.S.; Kim, S.J.; Choi, J.S.; Park, J.; Ju, S.M.; Han, S.J.; Kang, I.J.; Kang, Y.H. Bog blueberry anthocyanins alleviate photoaging in ultraviolet-B irradiation-induced human dermal fibroblasts. Mol. Nutr. Food Res. 2009, 53, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Tulipani, S.; Gonzales-Paramas, A.M.; Santos-Buelga, C.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Photoprotective potential of strawberry (Fragaria × ananassa) extract against UV-A irradiation damage on human fibroblasts. J. Agric. Food Chem. 2012, 60, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Nrf2—A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Tarangini, K.; Mishra, S. Production, Characterization and Analysis of Melanin from Isolated Marine Pseudomonas sp. using Vegetable waste. Res. J. Eng. Sci. 2013, 2, 40–46. [Google Scholar]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hebert, N. Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigment Cell Res. 2006, 19, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K.; Park, J.S.; Kato, F.; Hirosaki, K.; Toyofuku, K.; Hua, C.; Yamashita, T. Assembly, Target-Signaling and Intracellular Transport of Tyrosinase Gene Family Proteins in the Initial Stage of Melanosome Biogenesis. Pigment Cell Res. 2000, 13, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Pauli, G.F.; Chen, S.N. Phytochemistry and biological properties of glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz Mohammadi, R.; Arablou, T. Resveratrol and endometriosis: In vitro and animal studies and underlying mechanisms. Biomed. Pharmacother. 2017, 91, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ruiz, C.V.; Ballesta de Los Santos, M.; Berna, J.; Fenoll, J.; Garcia-Ruiz, P.A.; Tudela, J.; Garcia-Canovas, F. Kinetic characterization of oxyresveratrol as a tyrosinase substrate. IUBMB Life 2015, 67, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, G.; Vielhaber, G.; Jacobs, K.; Franke, H. 4-(1-Phenylethyl)1,3-benzenediol: A new highly potent lightening agent. J. Cosmet. Sci. 2006, 57, 197–198. [Google Scholar] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Skroza, D.; Mekinić, I.G.; Svilović, S.; Šimat, V.; Katalinić, V. Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. J. Food Compos. Anal. 2015, 38, 13–18. [Google Scholar] [CrossRef]
- Mishra, G.P.; Sharma, R. Identification of Potential PPAR gamma Agonists as Hypoglycemic Agents: Molecular Docking Approach. Interdiscip. Sci. 2016, 8, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, Q.; Chen, X.; Xia, K.; Tang, J.; Zhou, X.; Cheng, Y.; Chen, Y.; Huang, L.; Xiang, H. Analysis of lncRNAs expression in UVB-induced stress responses of melanocytes. J. Dermatol. Sci. 2016, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Kushwaha, H.N.; Srivastava, A.K.; Srivastava, S.; Jamal, N.; Srivastava, K.; Ray, R.S. Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway: An application for photoprotection. J. Photochem. Photobiol. B Biol. 2017, 172, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Shan, C.; Liu, S.; Zheng, H.; Liu, C.; Liu, M.; Jin, F.; Wang, L. Skin resistance to UVB-induced oxidative stress and hyperpigmentation by the topical use of Lactobacillus helveticus NS8-fermented milk supernatant. J. Appl. Microbiol. 2017, 123, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 1970, 326, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Kurin, E.; Mučaji, P.; Nagy, M. In vitro antioxidant activities of three red wine polyphenols and their mixtures: An interaction study. Molecules 2012, 17, 14336–16348. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Roychowdhury, S.; Engelmann, M.; Wolf, G.; Wolf, G.; Horn, T.F.W. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: Effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide 2003, 9, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.H.; Shi, Y.R.; Choi, E.J.; Kang, S.H.; Chang, I.M.; Min, K.R.; Kim, Y. Oxyresveratrol as the Potent Inhibitor on Dopa Oxidase Activity of Mushroom Tyrosinase. Biochem. Biophys. Res. Commun. 1998, 243, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Kubo, I. Resveratrol as a kcat type inhibitor for tyrosinase: Potentiated melanogenesis inhibitor. Bioorg. Med. Chem. 2012, 20, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.; Seo, I.; Liebel, F.; Southall, M.D.; Kollias, N.; Ruvolo, E. Visible Light Induces Melanogenesis in Human Skin through a Photoadaptive Response. PLoS ONE 2015, 10, e0130949. [Google Scholar] [CrossRef] [PubMed]
- Che, D.N.; Xie, G.H.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Protective effects of grape stem extract against UVB-induced damage in C57BL mice skin. J. Photochem. Photobiol. B 2017, 173, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Nandar, S.K.; Kathuria, S.; Ramesh, V. Effects of air pollution on the skin: A review. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 415–423. [Google Scholar] [PubMed]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Eumelanin-driven production of molecular hydrogen: A novel element of skin defense? Med. Hypotheses 2015, 85, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Dinkovakostova, A.T.; Kazantsev, A.G. Activation of Nrf2 signaling as a common treatment of neurodegenerative diseases. Neurodegener. Dis. Manag. 2017, 7, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Soeur, J.; Eilstein, J.; Lereaux, G.; Jones, C.; Marrot, L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic. Biol. Med. 2015, 78, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Hyun, Y.J.; Hewage, S.; Piao, M.J.; Kang, K.A.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Hyun, J.W. 3-Bromo-4,5-dihydroxybenzaldehyde Enhances the Level of Reduced Glutathione via the Nrf2-Mediated Pathway in Human Keratinocytes. Mar. Drugs 2017, 15, 291. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lin, P.; Hwang, E.; Wang, Y.; Yan, Z.; Ngo, H.T.T.; Yi, T.H. Pterocarpus santalinus L. Regulated Ultraviolet B Irradiation-induced Procollagen Reduction and Matrix Metalloproteinases Expression through Activation of TGF-β/Smad and Inhibition of the MAPK/AP-1 Pathway in Normal Human Dermal Fibroblasts. Photochem. Photobiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ben Yehuda Greenwald, M.; Frusic-Zlotkin, M.; Soroka, Y.; Ben Sasson, S.; Bitton, R.; Bianco-Peled, H.; Kohen, R. Curcumin Protects Skin against UVB-Induced Cytotoxicity via the Keap1-Nrf2 Pathway: The Use of a Microemulsion Delivery System. Oxid. Med. Cell. Longev. 2017, 2017, 5205471. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kondo, R.; Sakai, K. Inhibition of tyrosinase by flavonoids, stilbenes and related 4-substituted resorcinols: Structure-activity investigations. Planta Medica 2000, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Westerhof, W.; Kooyers, T.J. Hydroquinone and its analogues in dermatology-a potential health risk. J. Cosmet. Dermatol. 2005, 4, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Kirkland, D.; Marzin, D.; Toutain, H.; Leclerc-Ribaud, C.; Jinnai, H. An assessment of the genotoxicity and human health risk of topical use of kojic acid[5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one]. Food Chem. Toxicol. 2004, 42, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Imai, T.; Onose, J.; Ueda, M.; Tamura, T.; Mitsumori, K.; Izumi, K.; Hirose, M. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl)nitrosamine or N-diethylnitrosamine. Toxicol. Sci. 2004, 81, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Kawai, K.; Kawai, K. Contact allergy to kojic acid in skin care products. Contact Dermat. 1995, 32, 9–13. [Google Scholar] [CrossRef]
- Tokura, Y.; Fujiyama, T.; Ikeya, S.; Tatsuno, K.; Aoshima, M.; Kasuya, A.; Ito, T. Biochemical, cytological, and immunological mechanisms of rhododendrol-induced leukoderma. J. Dermatol. Sci. 2015, 77, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Cheng, K.C.; Lin, Y.C.; Chen, C.Y.; Chou, H.Y.; Ma, D.L.; Leung, C.H.; Wen, Z.H.; Wang, H.M. Synergistic Effects of Linderanolide B Combined with Arbutin, PTU or Kojic Acid on Tyrosinase Inhibition. Curr. Pharm. Biotechnol. 2015, 16, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Lee, S.J.; Chung, M.H.; Park, J.H.; Park, Y.I.; Cho, T.H.; Lee, S.K. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch. Pharm. Res. 1999, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, P.A. Mechanistic aspects of the tyrosinase oxidation of hydroquinone. Bioorg. Med. Chem. Lett. 2014, 24, 2463–2464. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.X.; Song, K.K. Tyrosinase Recent Prospects. J. Xiamen Univ. Natl. Sci. 2006, 5, 731–737. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Samples | IC50 (µmol/L) |
|---|---|
| OXYR | 76 ± 0.07 |
| PR | 119 ± 0.07 |
| RES | 142 ± 0.03 |
| GLA | 458 ± 0.037 |
| Substance | IC50 (µmol/L) | |
|---|---|---|
| l-Tyrosine | l-DOPA | |
| GLA | 1.5 ± 0.03 | 6.0 ± 0.23 |
| OXYR | 2.5 ± 0.06 | 19.5 ± 0.07 |
| PR | 0.28 ± 0.03 | 35.5 ± 0.06 |
| RES | 23.0 ± 0.05 | 2.5 ± 0.28 |
| Compounds | Antioxidant Activity | Tyrosinase Inhibitory Activity | IC50mix(µmol/L) | IC50add(µmol/L) | CI |
|---|---|---|---|---|---|
| PR:GLA | PR > GLA | PR < GLA | 16.5 ± 0.04 | 1.08 ± 0.02 * | 2.03 ± 0.02 |
| GLA:RES | GLA < RES | GLA < RES | 18.5 ± 0.19 | 9.98 ± 0.05 * | 1.82 ± 0.02 |
| GLA:OXYR | GLA < OXYR | GLA > OXYR | 2.5 ± 0.28 | 2.05 ± 0.07 | 1.28 ± 0.05 |
| OXYR:RES | OXYR > RES | OXYR < RES | 12.5 ± 0.05 | 9.72 ± 0.05 * | 1.36 ± 0.04 |
| PR:OXYR | PR < OXYR | PR < OXYR | 2.15 ± 0.03 | 1.82 ± 0.10 | 1.18 ± 0.03 |
| PR:RES | PR > RES | PR < RES | 17.5 ± 0.28 | 12.75 ± 0.02 * | 1.35 ± 0.05 |
| Compounds | Antioxidant Activity | Tyrosinase Inhibitory Activity | IC50mix(µmol/L) | IC50add(µmol/L) | CI |
|---|---|---|---|---|---|
| PR:GLA | PR > GLA | PR < GLA | 18 ± 0.16 | 23 ± 0.01 * | 0.71 ± 0.04 |
| GLA:RES | GLA < RES | GLA < RES | 3.5 ± 0.09 | 3.3 ± 0.17 | 0.96 ± 0.08 |
| GLA:OXYR | GLA < OXYR | GLA > OXYR | 8.1 ± 0.03 | 15.5 ± 0.05 * | 0.57 ± 0.01 |
| OXYR:RES | OXYR > RES | OXYR < RES | 2.9 ± 0.16 | 3.9 ± 0.01 * | 0.75 ± 0.05 |
| PR:OXYR | PR < OXYR | PR < OXYR | 24 ± 0.03 | 25 ± 0.02 | 0.94 ± 0.06 |
| PR:RES | PR > RES | PR < RES | 6 ± 0.01 | 37 ± 0.02 * | 0.78 ± 0.02 |
| CDOCKER_INTERACTION ENERGY (Kcal/mol) | PR | OXYR | GLA | RES |
|---|---|---|---|---|
| 2Y9X | −30.41 | −35.77 | −36.57 | −37.53 |
| 4IQK | −20.22 | −33.37 | −26.35 | −30.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hao, M.-M.; Sun, Y.; Wang, L.-F.; Wang, H.; Zhang, Y.-J.; Li, H.-Y.; Zhuang, P.-W.; Yang, Z. Synergistic Promotion on Tyrosinase Inhibition by Antioxidants. Molecules 2018, 23, 106. https://doi.org/10.3390/molecules23010106
Wang Y, Hao M-M, Sun Y, Wang L-F, Wang H, Zhang Y-J, Li H-Y, Zhuang P-W, Yang Z. Synergistic Promotion on Tyrosinase Inhibition by Antioxidants. Molecules. 2018; 23(1):106. https://doi.org/10.3390/molecules23010106
Chicago/Turabian StyleWang, Yan, Mi-Mi Hao, Ying Sun, Li-Feng Wang, Hao Wang, Yan-Jun Zhang, Hong-Yan Li, Peng-Wei Zhuang, and Zhen Yang. 2018. "Synergistic Promotion on Tyrosinase Inhibition by Antioxidants" Molecules 23, no. 1: 106. https://doi.org/10.3390/molecules23010106





