Chiral Symmetry Breaking in Magnetoelectrochemical Etching with Chloride Additives
Abstract
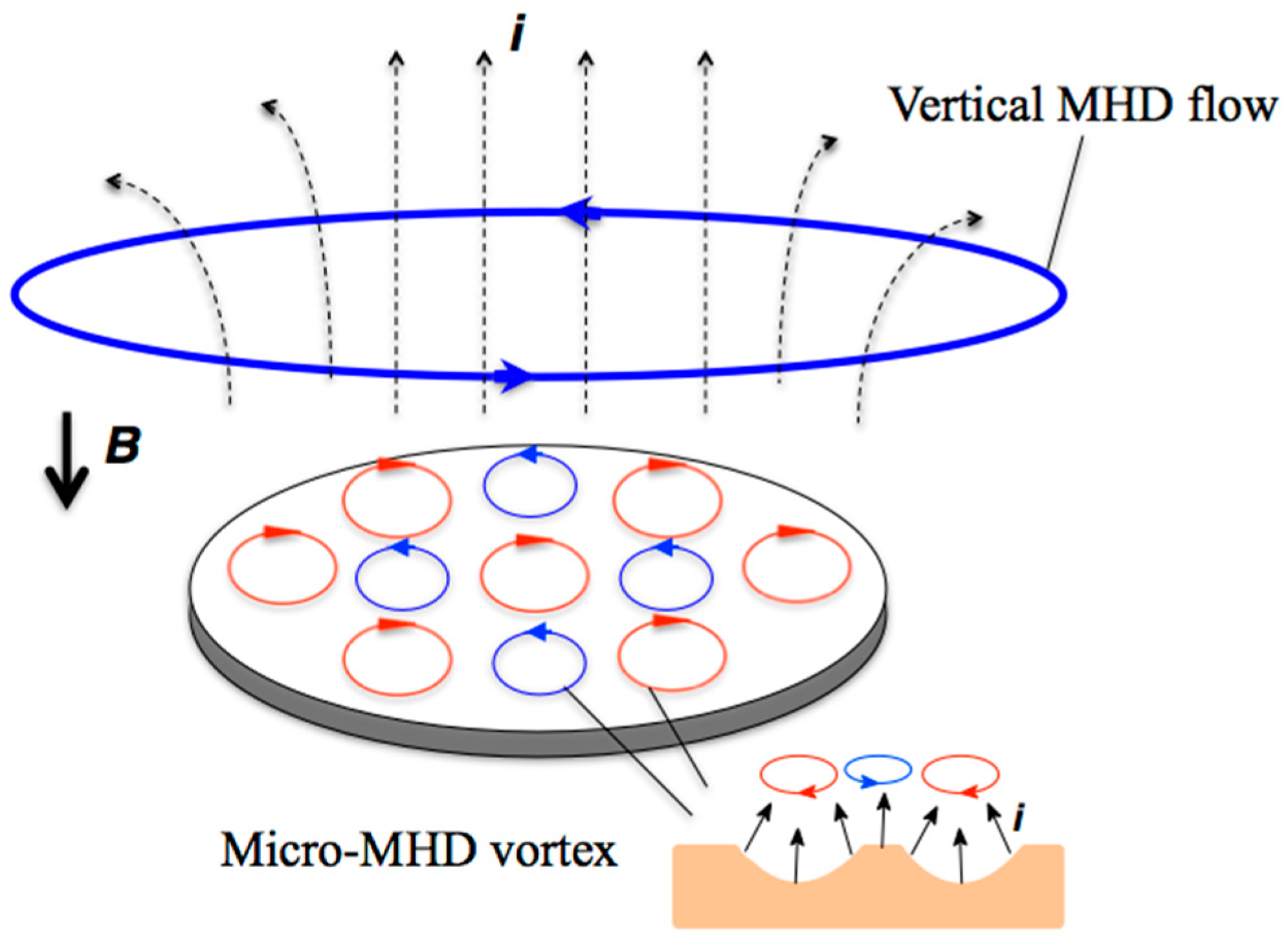
:1. Introduction
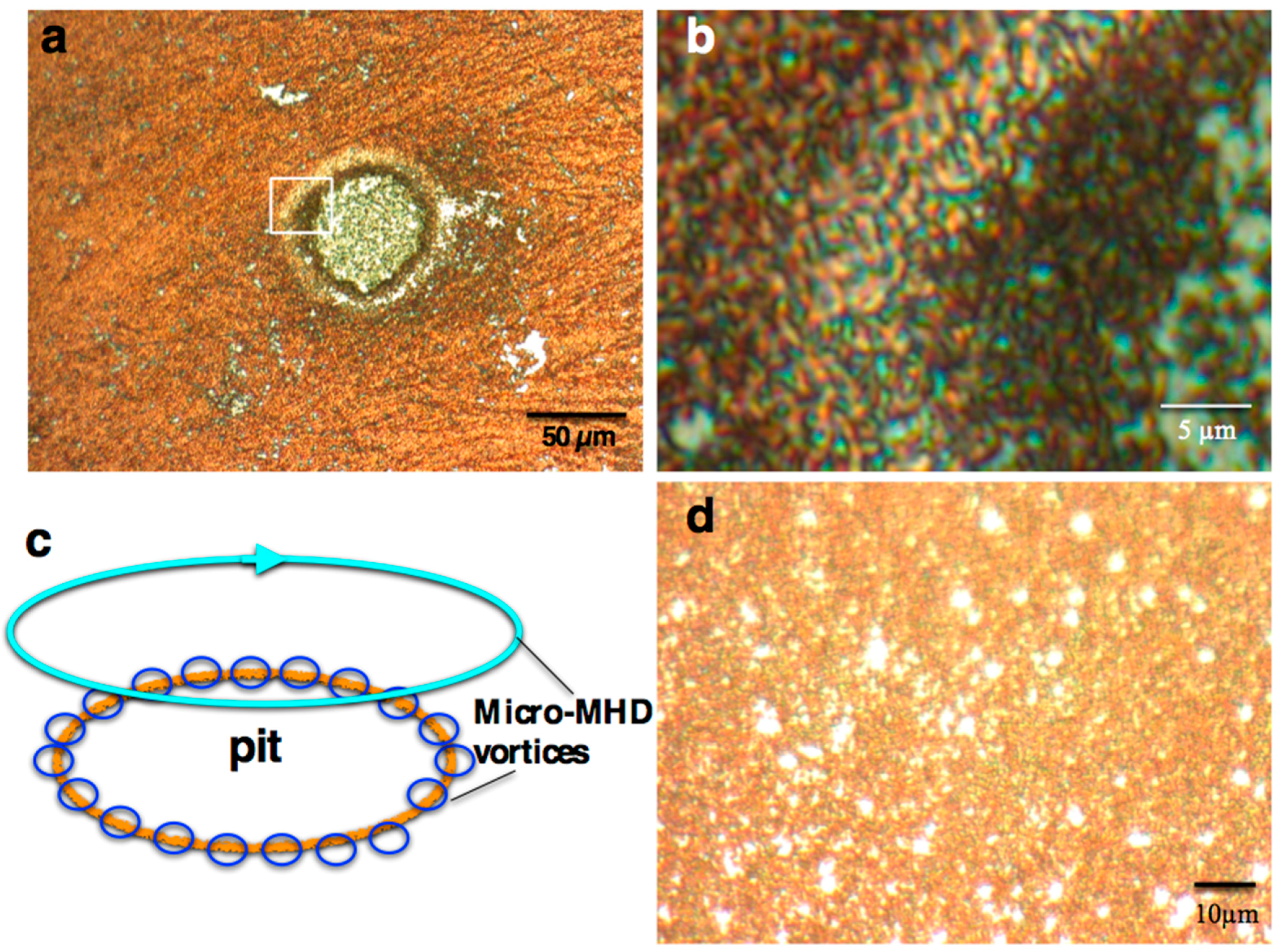
2. Results and Discussion
2.1. Surface Morphology of the MEE Films
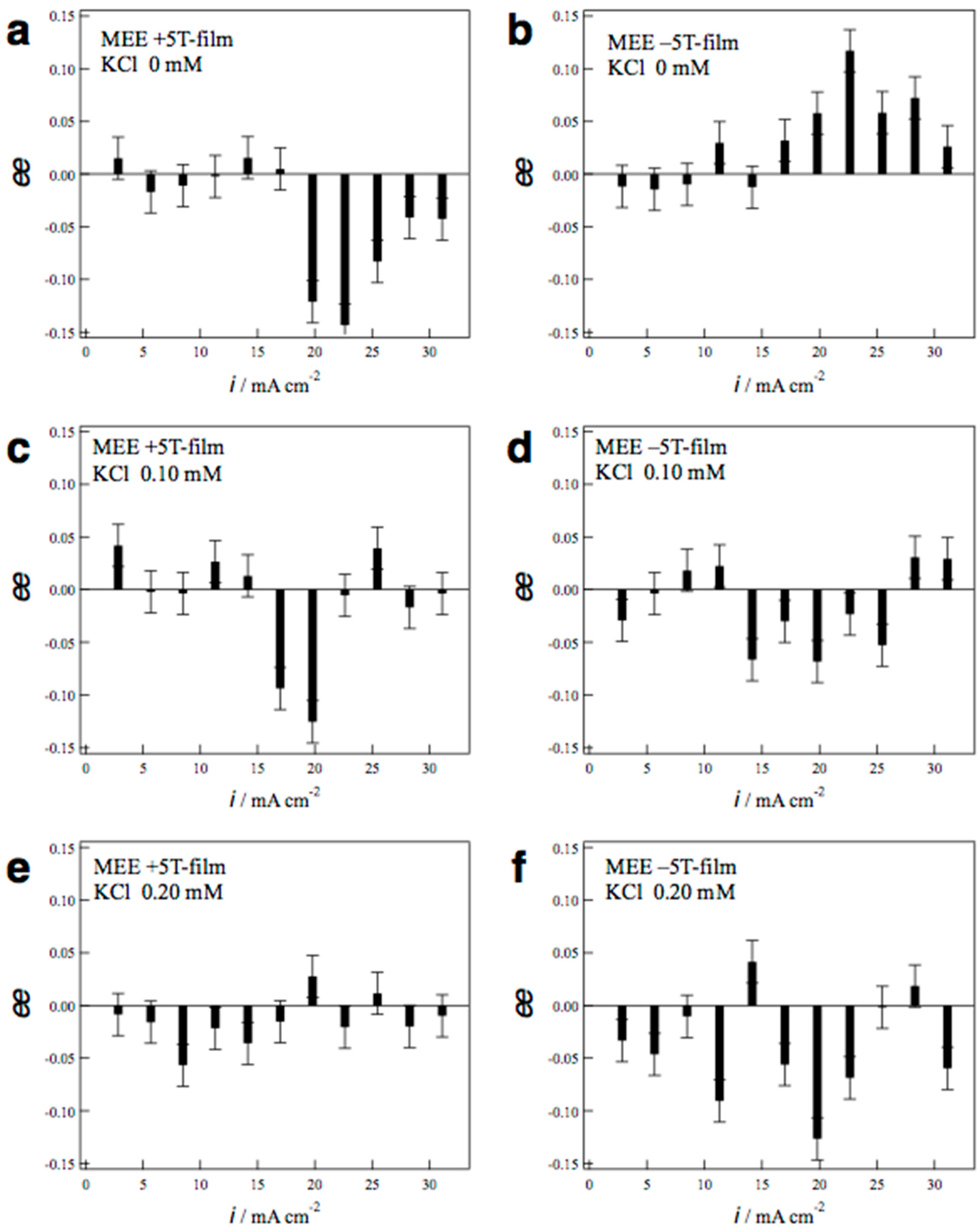
2.2. Surface Chirality of the MEE Films
3. Materials and Methods
3.1. Electrochemical Cell and Etching Procedures
3.2. MEE Procedures
3.3. Estimation of Surface Chirality
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamamoto, M.; Nakamura, R.; Kasaya, T.; Kumagai, H.; Suzuki, K.; Takai, K. Spontaneous and Widespread Electricity Generation in Natural Deep-Sea Hydrothermal Fields. Angew. Chem. Int. Ed. 2017, 56, 5725–5728. [Google Scholar] [CrossRef] [PubMed]
- Wachterchauser, G. Before Enzyme and Templates: Theory of Surface Metabolism. Microbiol. Rev. 1988, 52, 452–484. [Google Scholar]
- Mogi, I.; Watanabe, K. Chiral Electrode Behavior of Magneto-Electrodeposited Silver Films. ISIJ Int. 2007, 47, 585–587. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K. Chiral Recognition of Amino Acids by Magnetoelectrodeposited Cu Film Electrodes. Int. J. Electrochem. 2011, 2011, 239637. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K. Enantioselective Recognition of Tartaric Acid on Magnetoelectrodeposited Copper Film Electrodes. Chem. Lett. 2012, 41, 1439–1441. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Watanabe, K. Chiral Surface Formation of Copper Films by Magnetoelectrochemical Etching. Magnetohydrodynamics 2015, 51, 361–368. [Google Scholar]
- Aogaki, R.; Morimoto, R. Nonequilibrium Fluctuations in Micro-MHD Effects on Electrodeposition. In Heat and Mass Transfer: Modeling and Simulation; Hossain, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 189–216. [Google Scholar]
- Sommeria, J.; Meyers, S.D.; Swinney, H.L. Laboratory Simulation of Jupiter’s Great Red Spot. Nature 1988, 331, 689–693. [Google Scholar] [CrossRef]
- Marcus, S.M. Numerical Simulation of Jupiter’s Great Red Spot. Nature 1988, 331, 693–696. [Google Scholar] [CrossRef]
- Rikken, G.L.J.A.; Folling, J.; Wyder, P. Electrical Magnetochiral Anisotropy. Phys. Rev. Lett. 2001, 87, 236602. [Google Scholar] [CrossRef] [PubMed]
- Mogi, I.; Aogaki, R.; Watanabe, K. Tailoring of Surface Chirality by Micro-Vortices and Specific Adsorption in Magnetoelectrodeposition. Bull. Chem. Soc. Jpn. 2015, 88, 1479–1485. [Google Scholar] [CrossRef]
- Aogaki, R. Micro-MHD Effect on Electrodeposition in Vertical Magnetic Field. Magnetohydrodynamics 2003, 4, 453–460. [Google Scholar]
- Shinohara, K.; Hashimoto, K.; Aogaki, R. Hexagonal Corrosion Pattern upon Cleavage of a Zinc Single Crystal under a Vertical High Magnetic Field. Chem. Lett. 2002, 31, 778–779. [Google Scholar] [CrossRef]
- Mogi, I.; Iwasaka, K.; Aogaki, R.; Takahashi, K. Visualization of Magnetohydrodynamic Micro-Vortices with Guanine Micro-Cryastals. J. Electrochem. Soc. 2017, 164, H584–H586. [Google Scholar] [CrossRef]
- Yanson, Y.I.; Rost, M.J. Structural Accelerating Effect of Chloride on Copper Electrodeposition. Angew. Chem. Int. Ed. 2013, 52, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Pattanaik, G.; Zangari, G. Influence of Chloride Anions on Mechanism of Copper Electrodeposition from Acidic Sulfate Electrolytes. J. Electrochem. Soc. 2007, 154, D201–D207. [Google Scholar] [CrossRef]
- Kao, Y.L.; Li, K.C.; Tu, G.C.; Huang, C.A. Microstructual Study of the Effect of Chloride Ion on Electroplating of Copper in Cupric Sulfate-Sulfuric Acid Bath. J. Electrochem. Soc. 2005, 152, C605–C611. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogi, I.; Aogaki, R.; Takahashi, K. Chiral Symmetry Breaking in Magnetoelectrochemical Etching with Chloride Additives. Molecules 2018, 23, 19. https://doi.org/10.3390/molecules23010019
Mogi I, Aogaki R, Takahashi K. Chiral Symmetry Breaking in Magnetoelectrochemical Etching with Chloride Additives. Molecules. 2018; 23(1):19. https://doi.org/10.3390/molecules23010019
Chicago/Turabian StyleMogi, Iwao, Ryoichi Aogaki, and Kohki Takahashi. 2018. "Chiral Symmetry Breaking in Magnetoelectrochemical Etching with Chloride Additives" Molecules 23, no. 1: 19. https://doi.org/10.3390/molecules23010019





