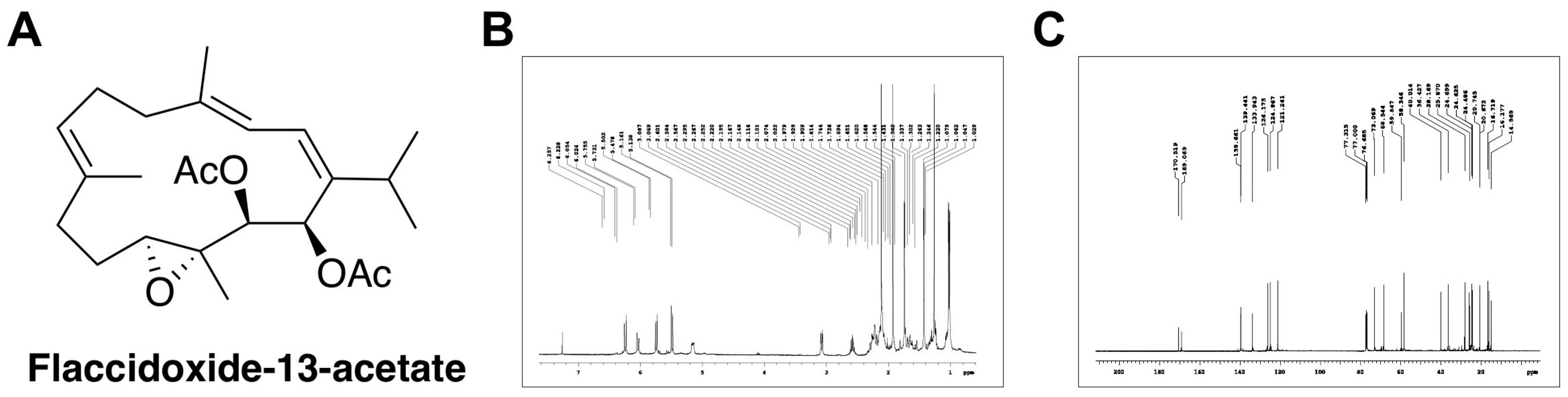
Flaccidoxide-13-Acetate Extracted from the Soft Coral Cladiella kashmani Reduces Human Bladder Cancer Cell Migration and Invasion through Reducing Activation of the FAK/PI3K/AKT/mTOR Signaling Pathway
Abstract
:1. Introduction
2. Results
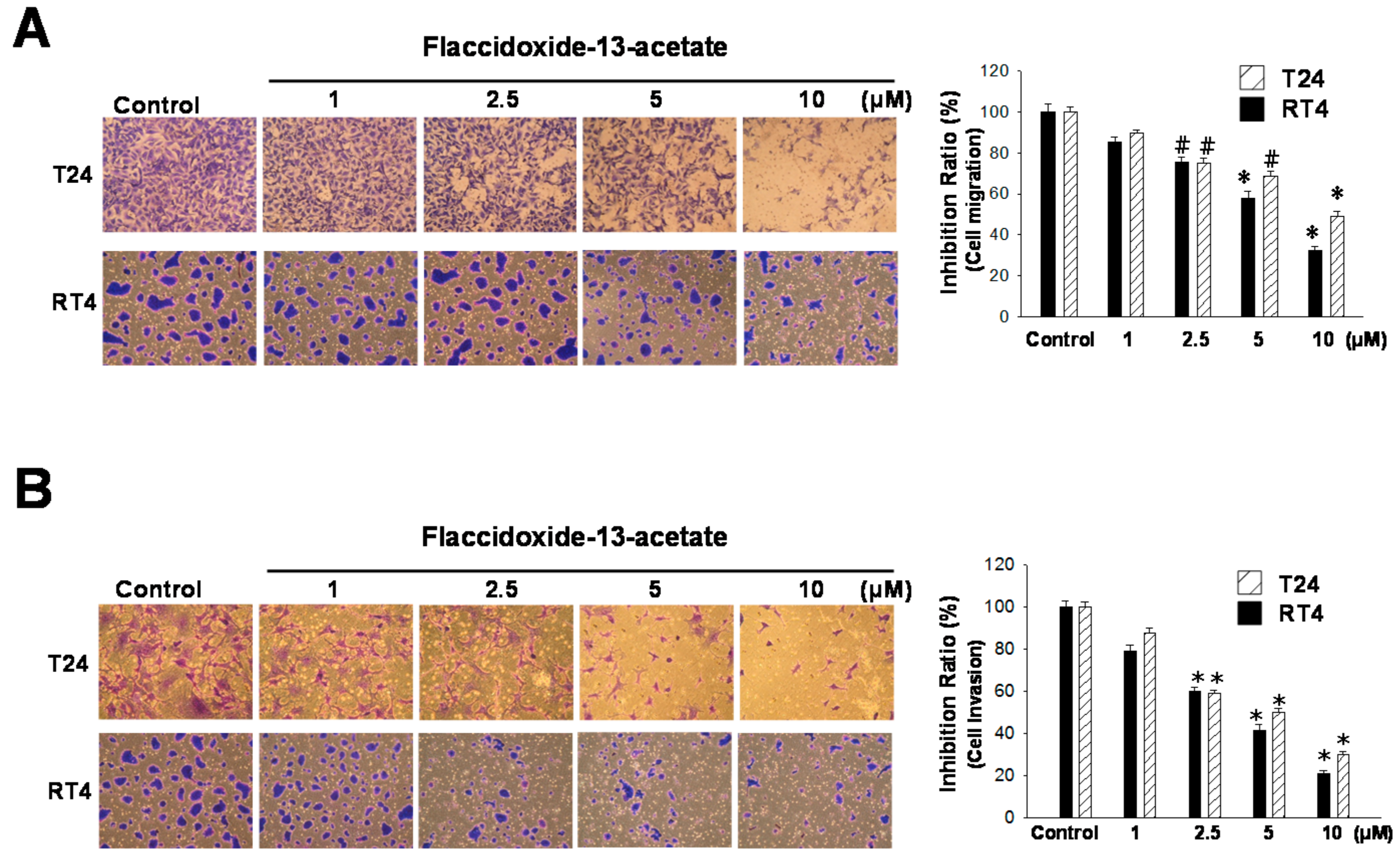
2.1. Inhibitory Effects of Flaccidoxide-13-Acetate on Cell Migration and Invasion
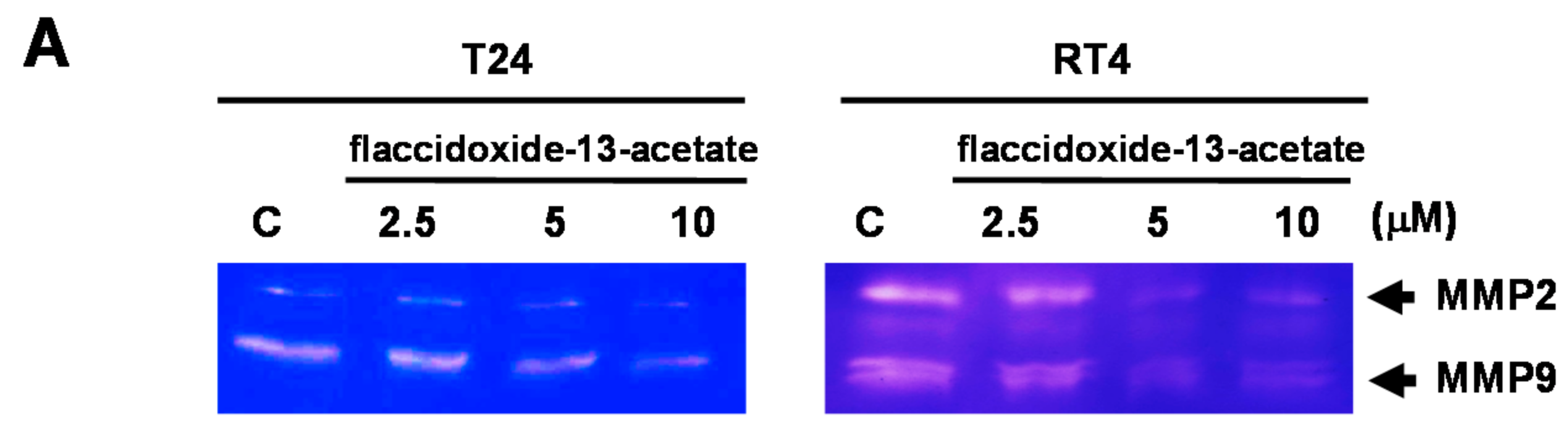
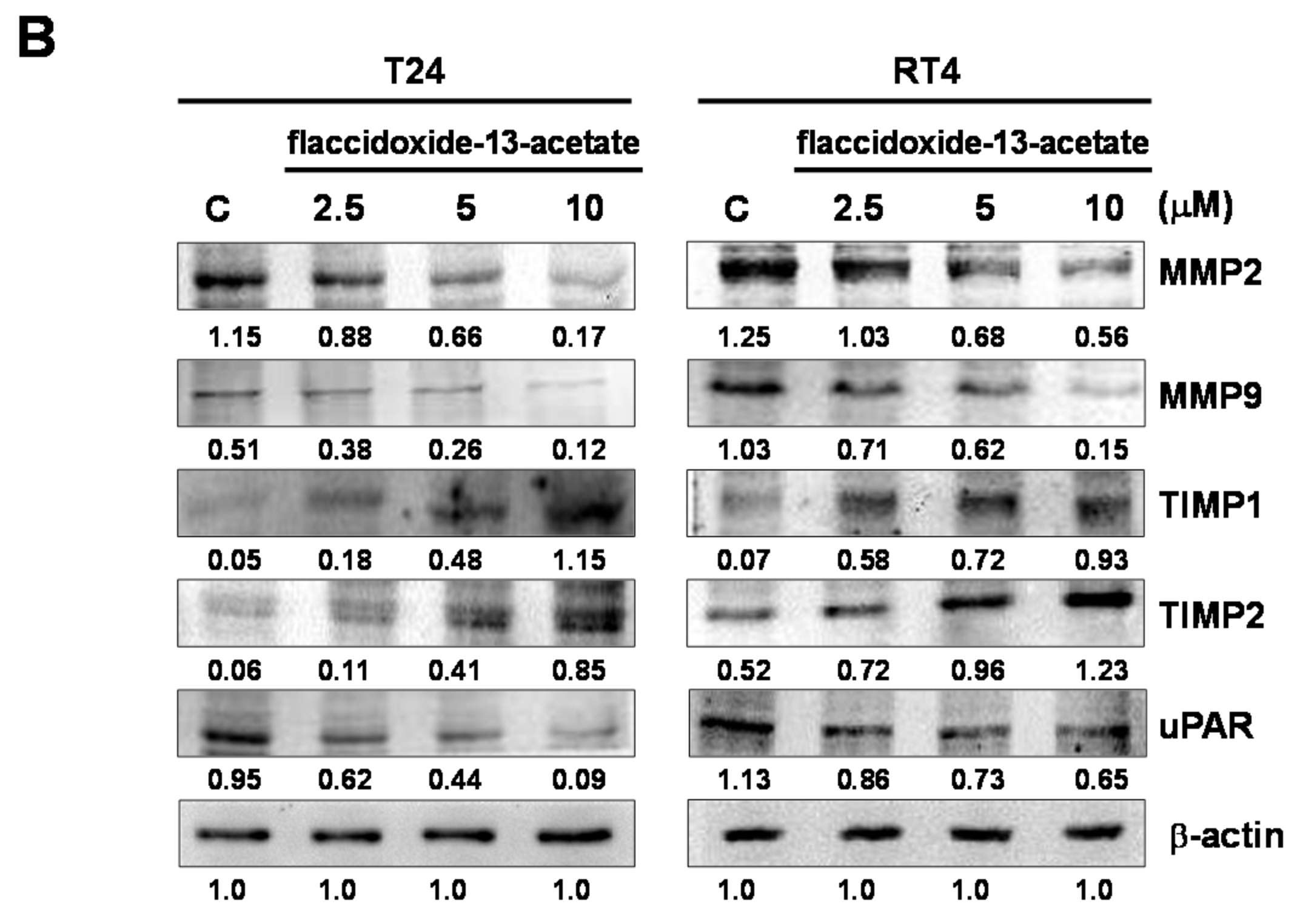
2.2. Effects of Flaccidoxide-13-Acetate on the Expression Levels of MMP-2, MMP-9, uPAR, TIMP-1 and TIMP-2
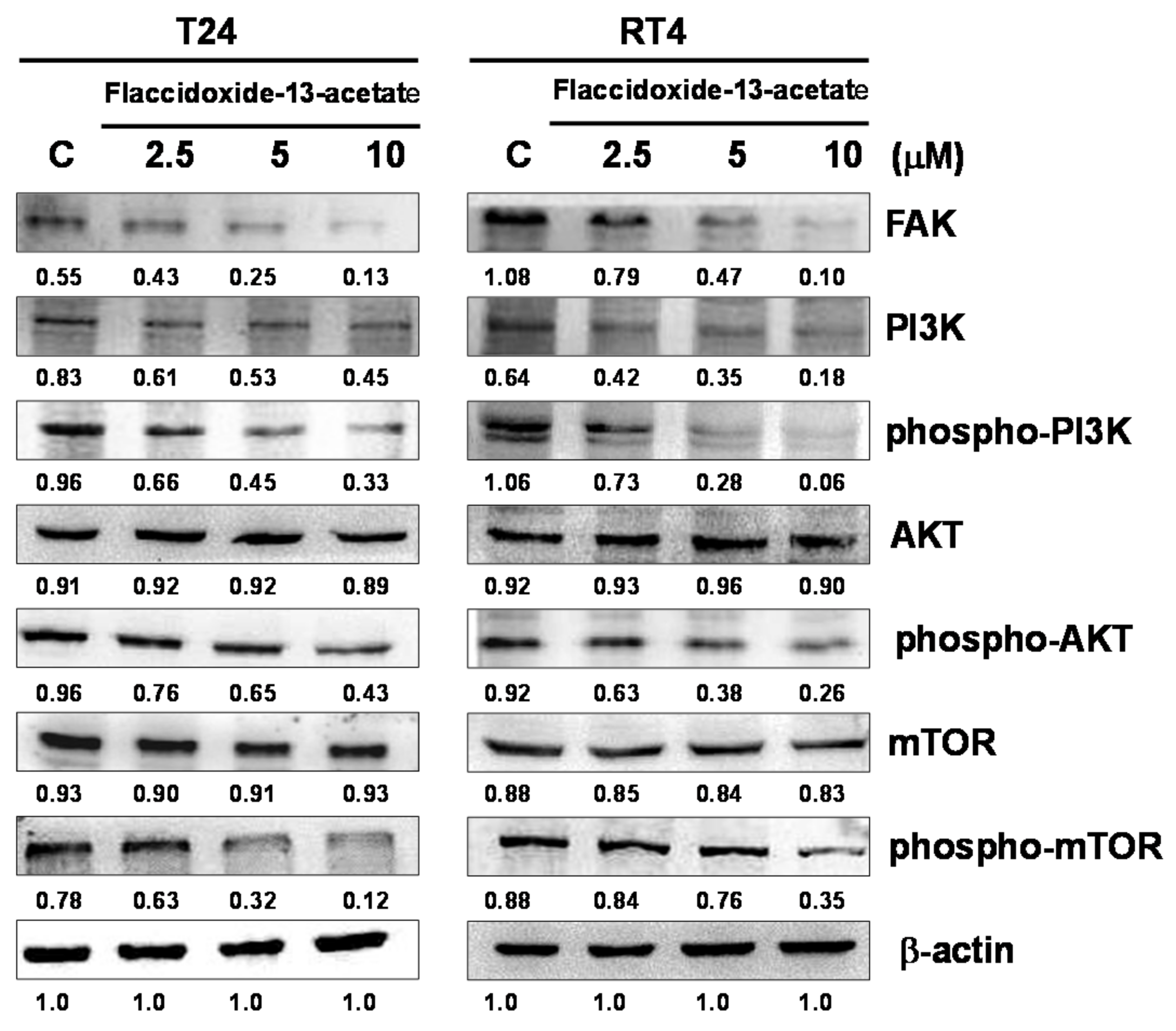
2.3. Effects of Flaccidoxide-13-Acetate on the FAK/PI3K/AKT/mTOR Signaling Pathway
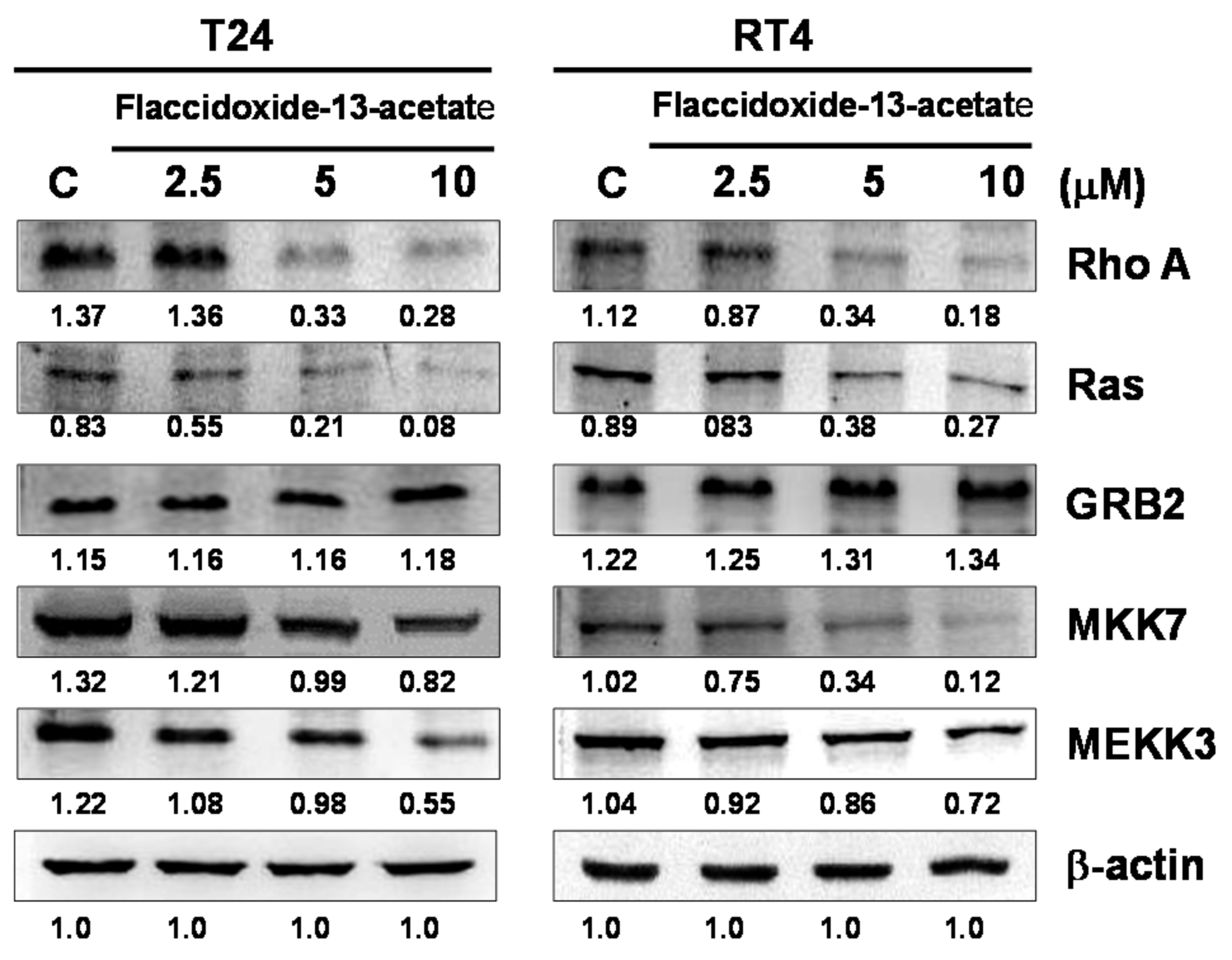
2.4. Effects of Flaccidoxide-13-Acetate on the Expressions of Cell Migration- and Invasion-Associated Proteins
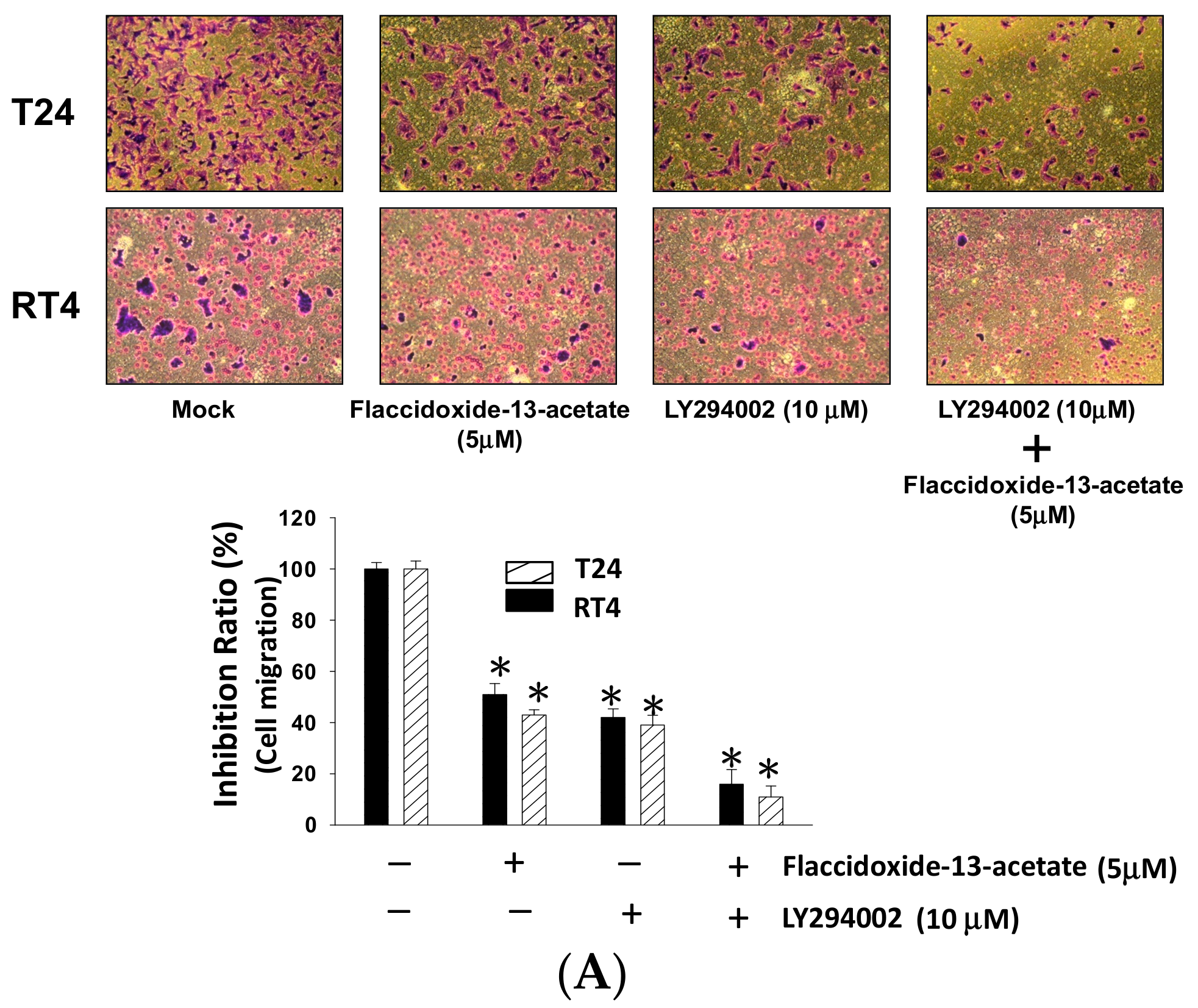
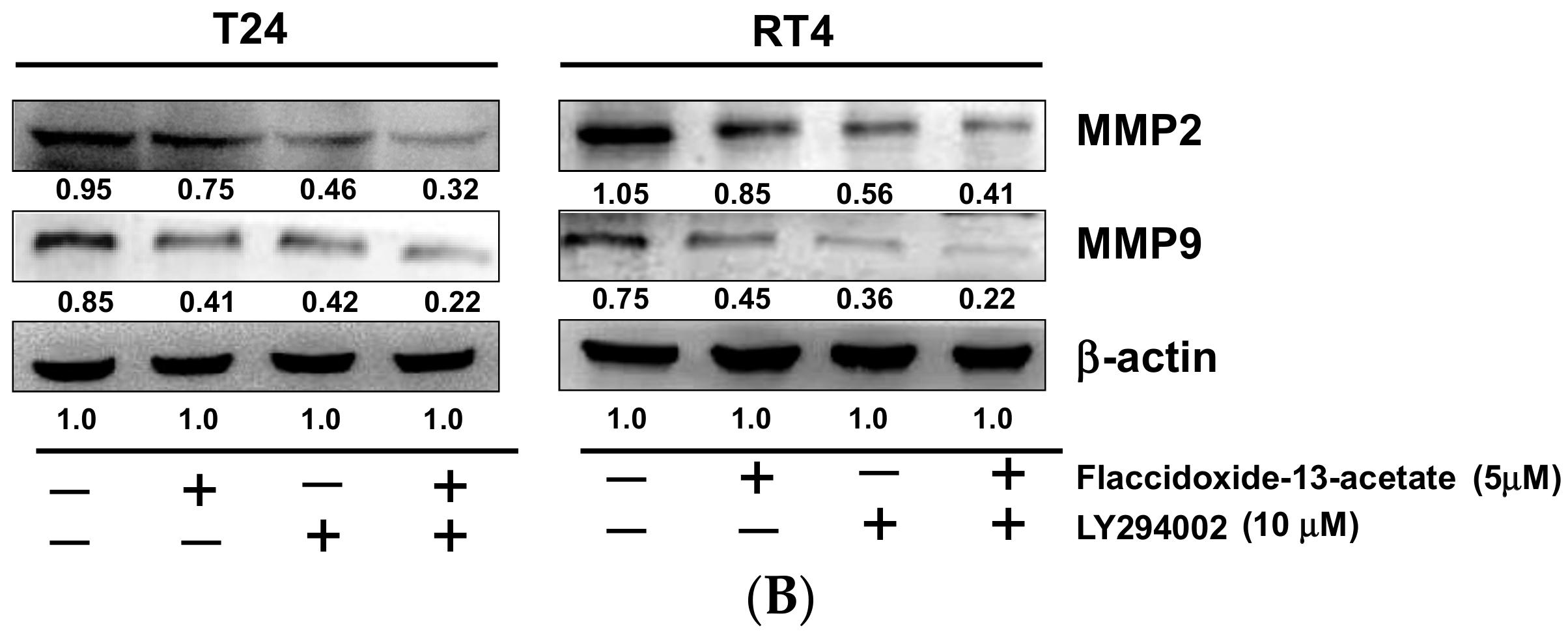
2.5. Inhibition of PI3K Reduced the Cell Migration and MMP-2 and MMP-9 Protein Expression
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. MTT Assay
4.4. Cell Migration and Invasion Assays
4.5. Determination of MMP-2/-9 Activities by Gelatin Zymography
4.6. Immunoblotting Analysis
4.7. Statistical Evaluation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peng, C.C.; Chen, K.C.; Peng, R.Y.; Su, C.H.; Hsieh-Li, H.M. Human urinary bladder cancer T24 cells are susceptible to the antrodia camphorata extracts. Cancer Lett. 2006, 243, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, A.; Nagaya, H.; Kanno, T.; Nishizaki, T. Antitumor action of α(1)-adrenoceptor blockers on human bladder, prostate and renal cancer cells. Pharmacology 2012, 90, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J. Recent advances in treatment of advanced urothelial carcinoma. Curr. Urol. Rep. 2012, 13, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Azemar, M.D.; Audouin, M.; Revaux, A.; Misrai, V.; Comperat, E.; Bitker, M.O.; Chartier-Kastler, E.; Richard, F.; Cussenot, O.; Roupret, M. Primary upper urinary tract tumors and subsequent location in the bladder. Prog. Urol. 2009, 19, 583–588. [Google Scholar] [PubMed]
- Dzombeta, T.; Krajacic-Jagarcec, G.; Tomas, D.; Kraus, O.; Ruzic, B.; Kruslin, B. Urothelial carcinoma with an inverted growth pattern: A report of 4 cases. Acta Med. Croat. 2010, 64, 47–50. [Google Scholar]
- Sun, H.Z.; Wu, S.F.; Tu, Z.H. Blockage of igf-1r signaling sensitizes urinary bladder cancer cells to mitomycin-mediated cytotoxicity. Cell Res. 2001, 11, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, K.; Zhang, J.; Chen, J.; Zhang, N.; Xu, Z. Relationship between patient age and superficial transitional cell carcinoma characteristics. Urology 2008, 71, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, J.H.; Young, R.H. Transitional cell carcinoma of the ovary: A morphologic study of 100 cases with emphasis on differential diagnosis. Am. J. Surg. Pathol. 2004, 28, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.A. Cancer. The metastasis cascade. Science 2008, 321, 1785–1787. [Google Scholar] [CrossRef] [PubMed]
- Amling, C.L. Diagnosis and management of superficial bladder cancer. Curr. Probl. Cancer 2001, 25, 219–278. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Dvoracek, J. Diagnosis and therapy of superficial tumors of the urinary bladder. Cas. Lek. Cesk. 2002, 141, 723–728. [Google Scholar] [PubMed]
- Ichigo, S.; Takagi, H.; Matsunami, K.; Murase, T.; Ikeda, T.; Imai, A. Transitional cell carcinoma of the ovary (review). Oncol. Lett. 2012, 3, 3–6. [Google Scholar] [PubMed]
- Wang, Z.; Lu, T.; Du, L.; Hu, Z.; Zhuang, Q.; Li, Y.; Wang, C.Y.; Zhu, H.; Ye, Z. Plasmacytoid urothelial carcinoma of the urinary bladder: A clinical pathological study and literature review. Int. J. Clin. Exp. Pathol. 2012, 5, 601–608. [Google Scholar] [PubMed]
- Chiang, A.C.; Massague, J. Molecular basis of metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Radhika, P. Chemical constituents and biological activities of the soft corals of genus cladiella: A review. Biochem. Syst. Ecol. 2006, 34, 781–789. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lan, Y.H.; Hsieh, P.W.; Wu, C.C.; Chen, S.L.; Yen, C.T.; Chang, F.R.; Hung, W.C.; Wu, Y.C. Bioactive cembrane diterpenoids of anisomeles indica. J. Nat. Prod. 2008, 71, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zeng, Y.; Ma, B.; Bi, K.; Chen, X. A new cytotoxic cembrane diterpene from the roots of euphorbia pekinensis rupr. Fitoterapia 2013, 90, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Li, G.Y.; Pu, J.X.; Xiao, W.L.; Ding, L.S.; Sun, H.D. Ent-kaurane and cembrane diterpenoids from isodon sculponeatus and their cytotoxicity. J. Nat. Prod. 2009, 72, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, C.; Liaw, C.; Chen, Y.; Kuo, Y.; Shen, Y. Cembrane diterpenoids from the taiwanese soft coral Sinularia flexibilis. Tetrahedron 2009, 65, 9157–9164. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Wu, M.L.; Hwang, Y.C.; Chang, F.R.; Wu, Y.C.; Wu, C.C. The natural diterpenoid ovatodiolide induces cell cycle arrest and apoptosis in human oral squamous cell carcinoma ca9-22 cells. Life Sci. 2009, 85, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Chen, Y.C.; El-Shazly, M.; Du, Y.C.; Su, J.H.; Tsao, C.W.; Yen, W.H.; Chang, W.B.; Su, Y.D.; Yeh, Y.T.; et al. 5-episinuleptolide acetate, a norcembranoidal diterpene from the formosan soft coral Sinularia sp., induces leukemia cell apoptosis through hsp90 inhibition. Molecules 2013, 18, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.A.; Davies-Coleman, M.T.; Schleyer, M.H. Cembrane diterpenes from the southern African soft coral Cladiella kashmani. J. Nat. Prod. 2000, 63, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 1999, 43, S42–S51. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Tanioka, M.; Matsuda, H.; Nishimoto, H.; Yoshioka, T.; Suzuki, R.; Uehira, M. Experimental metastasis is suppressed in mmp-9-deficient mice. Clin. Exp. Metastasis 1999, 17, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Takahashi, H.; Murai, Y.; Cui, Z.; Nomoto, K.; Niwa, H.; Tsuneyama, K.; Takano, Y. Expressions of mmp-2, mmp-9 and vegf are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006, 26, 3579–3583. [Google Scholar] [PubMed]
- Gentner, B.; Wein, A.; Croner, R.S.; Zeittraeger, I.; Wirtz, R.M.; Roedel, F.; Dimmler, A.; Dorlaque, L.; Hohenberger, W.; Hahn, E.G.; et al. Differences in the gene expression profile of matrix metalloproteinases (mmps) and their inhibitors (timps) in primary colorectal tumors and their synchronous liver metastases. Anticancer Res. 2009, 29, 67–74. [Google Scholar] [PubMed]
- Andreasen, P.A.; Kjoller, L.; Christensen, L.; Duffy, M.J. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int. J. Cancer 1997, 72, 1–22. [Google Scholar] [CrossRef]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Brouxhon, S.M.; Kyrkanides, S.; Teng, X.; Athar, M.; Ghazizadeh, S.; Simon, M.; O’Banion, M.K.; Ma, L. Soluble e-cadherin: A critical oncogene modulating receptor tyrosine kinases, mapk and pi3k/akt/mtor signaling. Oncogene 2014, 33, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Nan, K.J.; Guo, H.; Wang, W.J.; Ruan, Z.P.; Wang, S.H.; Liang, X.; Lu, C.X. Pten inhibits the migration and invasion of hepg2 cells by coordinately decreasing mmp expression via the pi3k/akt pathway. Oncol. Rep. 2010, 23, 1593–1600. [Google Scholar] [PubMed]
- Yang, N.; Hui, L.; Wang, Y.; Yang, H.; Jiang, X. Sox2 promotes the migration and invasion of laryngeal cancer cells by induction of mmp-2 via the pi3k/akt/mtor pathway. Oncol. Rep. 2014, 31, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Neoh, C.A.; Tsao, C.Y.; Su, J.H.; Li, H.H. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through mapks and pi3k/akt signaling pathways. Int. J. Mol. Sci. 2015, 16, 16469–16482. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Su, C.C.; Lu, H.F.; Yang, J.S.; Hsu, S.C.; Ip, S.W.; Wu, J.J.; Li, Y.C.; Ho, C.C.; Wu, C.C.; et al. Curcumin blocks migration and invasion of mouse-rat hybrid retina ganglion cells (n18) through the inhibition of mmp-2, -9, fak, rho a and rock-1 gene expression. Oncol. Rep. 2010, 23, 665–670. [Google Scholar] [PubMed]
- Abecassis, I.; Olofsson, B.; Schmid, M.; Zalcman, G.; Karniguian, A. Rhoa induces mmp-9 expression at cd44 lamellipodial focal complexes and promotes hmec-1 cell invasion. Exp. Cell Res. 2003, 291, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Thant, A.A.; Sein, T.T.; Liu, E.; Machida, K.; Kikkawa, F.; Koike, T.; Seiki, M.; Matsuda, S.; Hamaguchi, M. Ras pathway is required for the activation of mmp-2 secretion and for the invasion of src-transformed 3y1. Oncogene 1999, 18, 6555–6563. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.N.; Liu, Z.; Rafi, M.M. A diarylheptanoid from lesser galangal (alpinia officinarum) inhibits proinflammatory mediators via inhibition of mitogen-activated protein kinase, p44/42, and transcription factor nuclear factor-kappa b. J. Pharmacol. Exp. Ther. 2003, 305, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.A.; Wang, R.Y.; Din, Z.H.; Su, J.H.; Chen, Y.K.; Tsai, F.J.; Weng, S.H.; Wu, Y.J. Induction of apoptosis by sinulariolide from soft coral through mitochondrial-related and p38mapk pathways on human bladder carcinoma cells. Mar. Drugs 2012, 10, 2893–2911. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.B.; Hsieh, M.J.; Hsieh, Y.S.; Chien, M.H.; Lin, P.Y.; Chiou, H.L.; Yang, S.F. Terminalia catappa exerts antimetastatic effects on hepatocellular carcinoma through transcriptional inhibition of matrix metalloproteinase-9 by modulating nf-kappab and ap-1 activity. Evid. Based Complement. Alternat. Med. 2012, 2012, 595292. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, S.; Ji, Y.; Li, J.; An, P.; Ren, H.; Liang, R.; Yang, J.; Li, Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the erk pathway. PLoS ONE 2013, 8, e72927. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neoh, C.-A.; Wu, W.-T.; Dai, G.-F.; Su, J.-H.; Liu, C.-I.; Su, T.-R.; Wu, Y.-J. Flaccidoxide-13-Acetate Extracted from the Soft Coral Cladiella kashmani Reduces Human Bladder Cancer Cell Migration and Invasion through Reducing Activation of the FAK/PI3K/AKT/mTOR Signaling Pathway. Molecules 2018, 23, 58. https://doi.org/10.3390/molecules23010058
Neoh C-A, Wu W-T, Dai G-F, Su J-H, Liu C-I, Su T-R, Wu Y-J. Flaccidoxide-13-Acetate Extracted from the Soft Coral Cladiella kashmani Reduces Human Bladder Cancer Cell Migration and Invasion through Reducing Activation of the FAK/PI3K/AKT/mTOR Signaling Pathway. Molecules. 2018; 23(1):58. https://doi.org/10.3390/molecules23010058
Chicago/Turabian StyleNeoh, Choo-Aun, Wen-Tung Wu, Guo-Fong Dai, Jui-Hsin Su, Chih-I Liu, Tzu-Rong Su, and Yu-Jen Wu. 2018. "Flaccidoxide-13-Acetate Extracted from the Soft Coral Cladiella kashmani Reduces Human Bladder Cancer Cell Migration and Invasion through Reducing Activation of the FAK/PI3K/AKT/mTOR Signaling Pathway" Molecules 23, no. 1: 58. https://doi.org/10.3390/molecules23010058



