Characterization of Vasorelaxant Principles from the Needles of Pinus morrisonicola Hayata
Abstract
:1. Introduction
2. Results
2.1. Phenolic Composition in 5LP Needle Extract
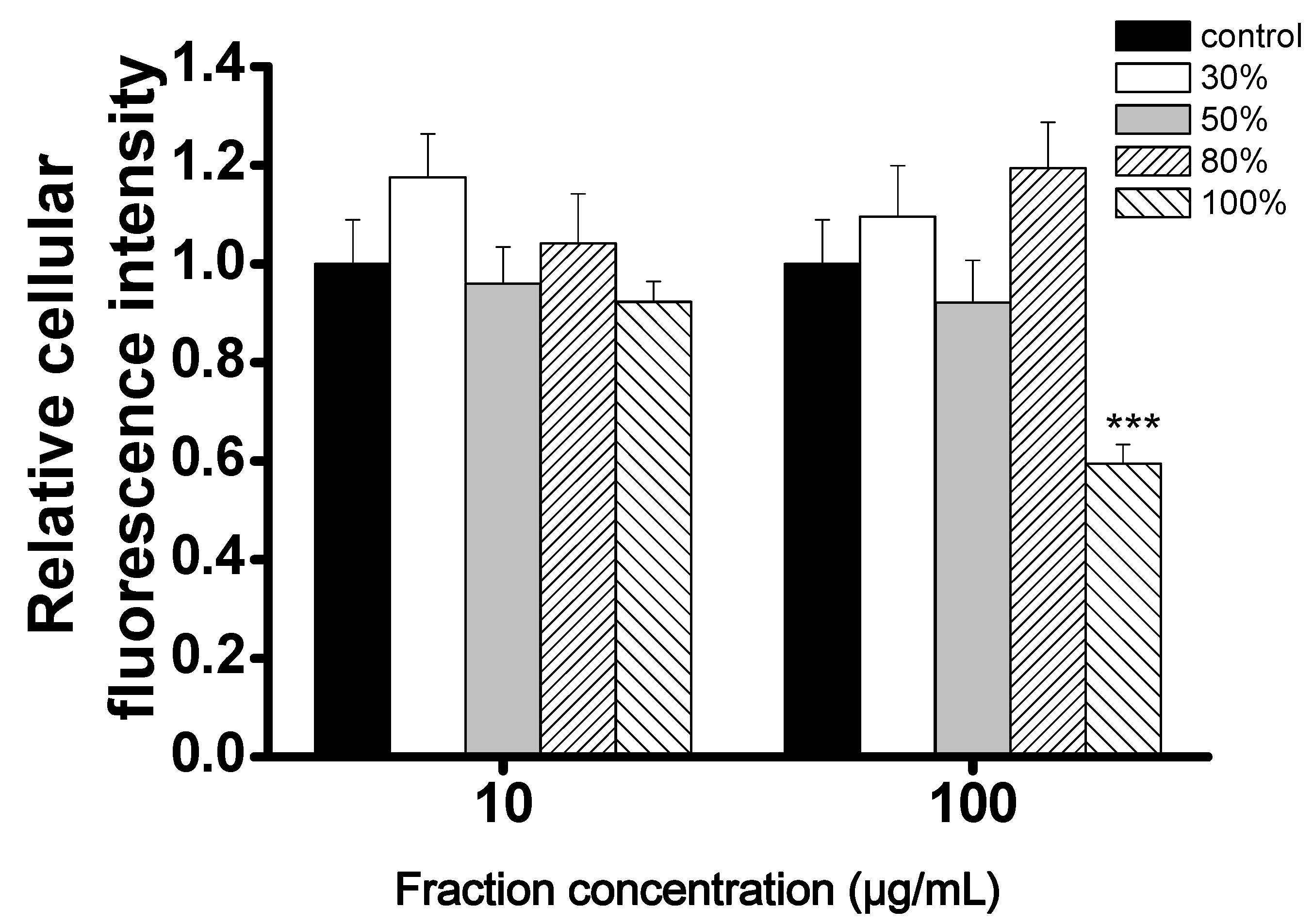
2.2. Evaluation of the CCB Candidates from the 5LP Fractions
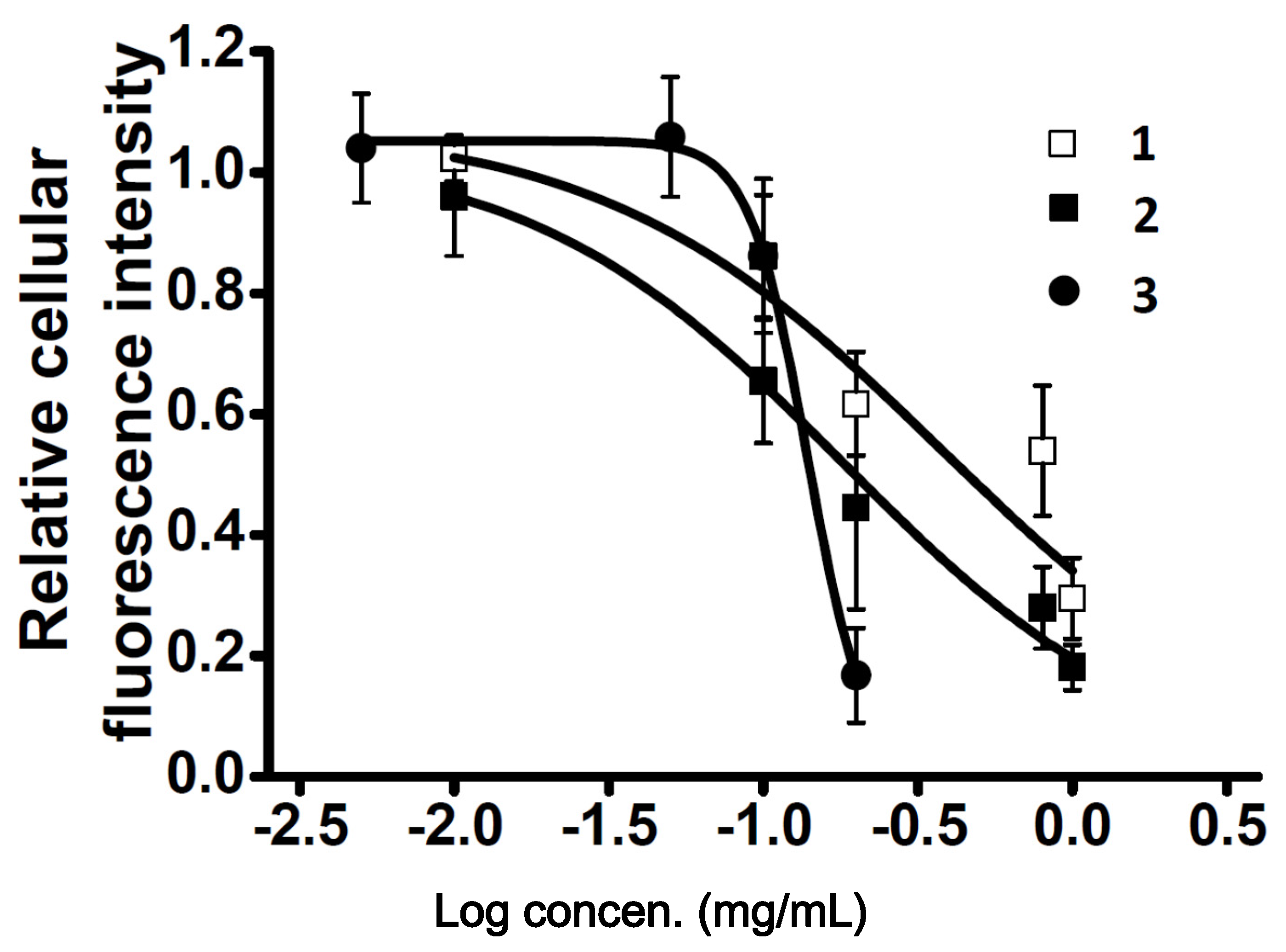
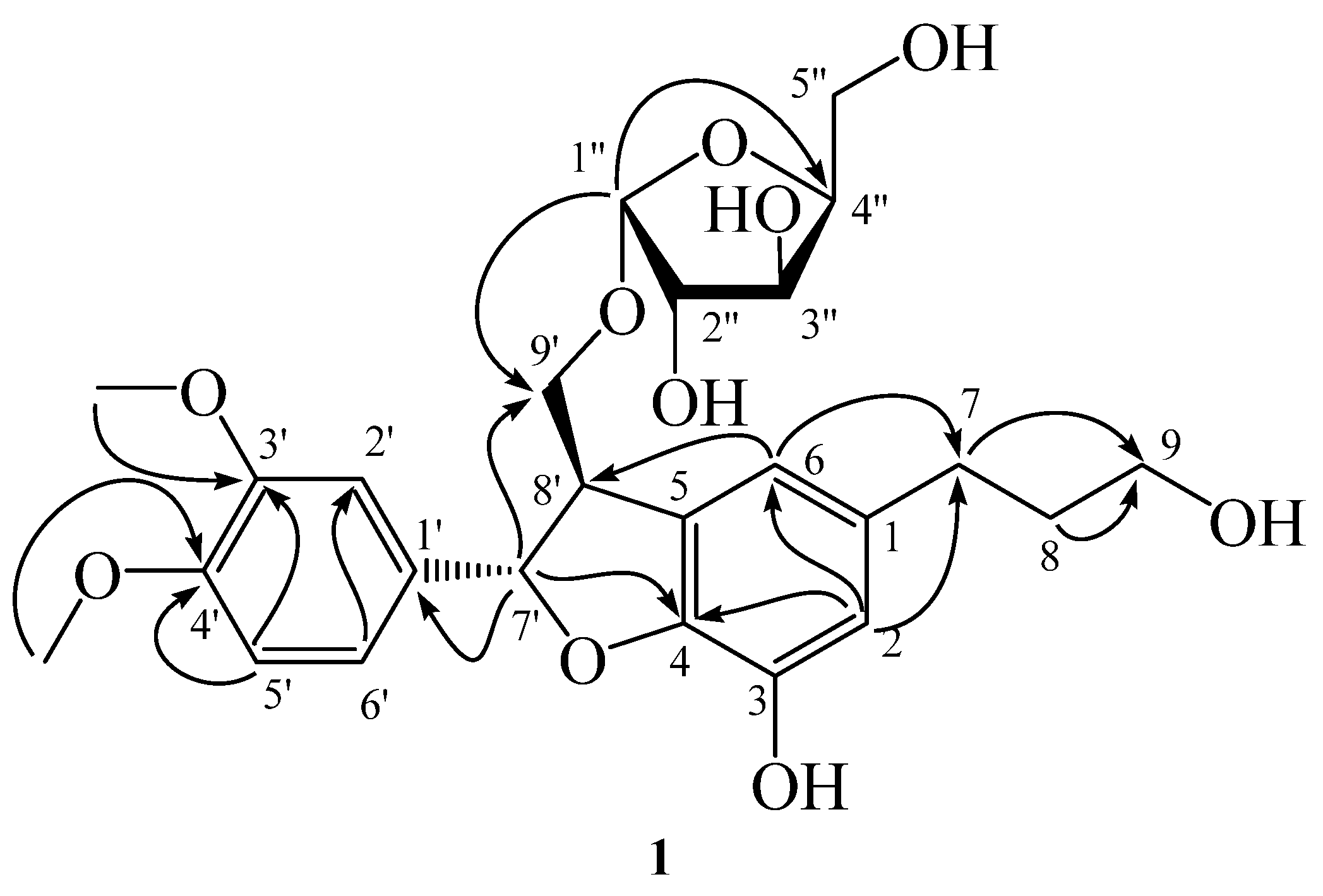
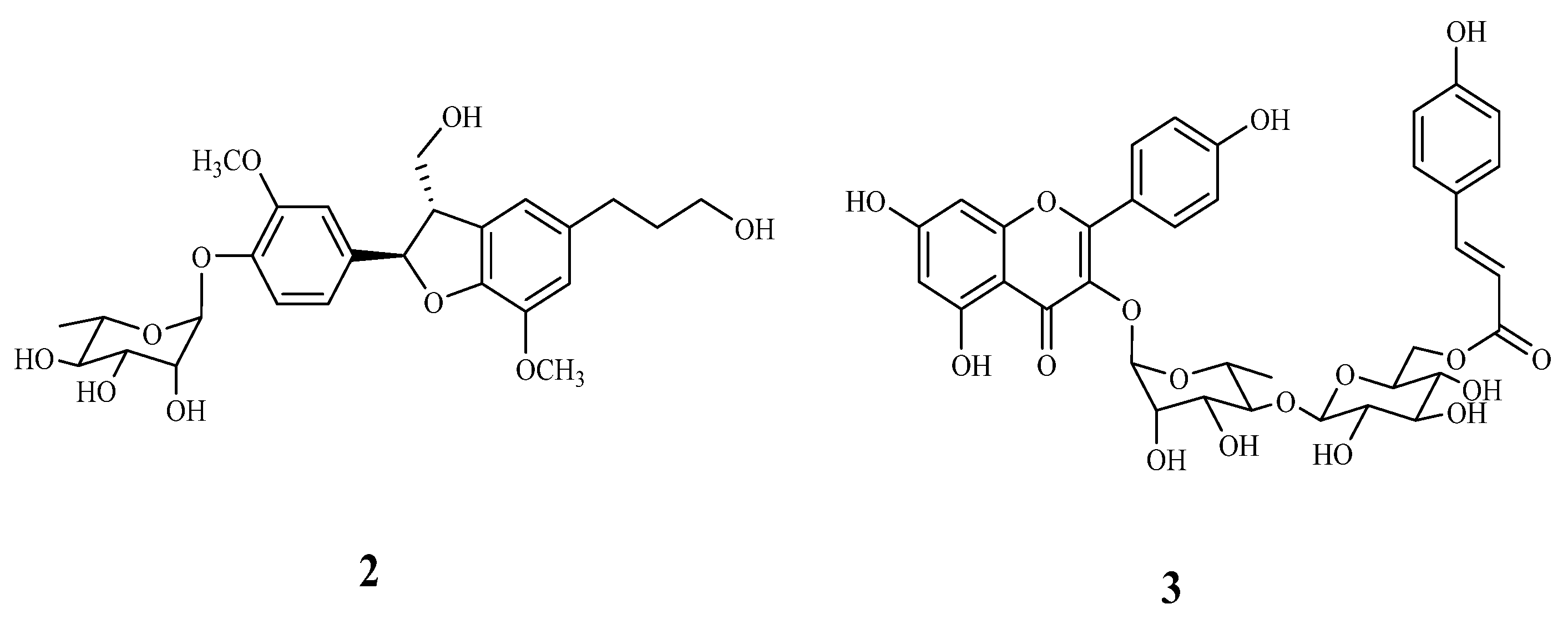
2.3. Characterization of the Vasodilation Candidates 1–3
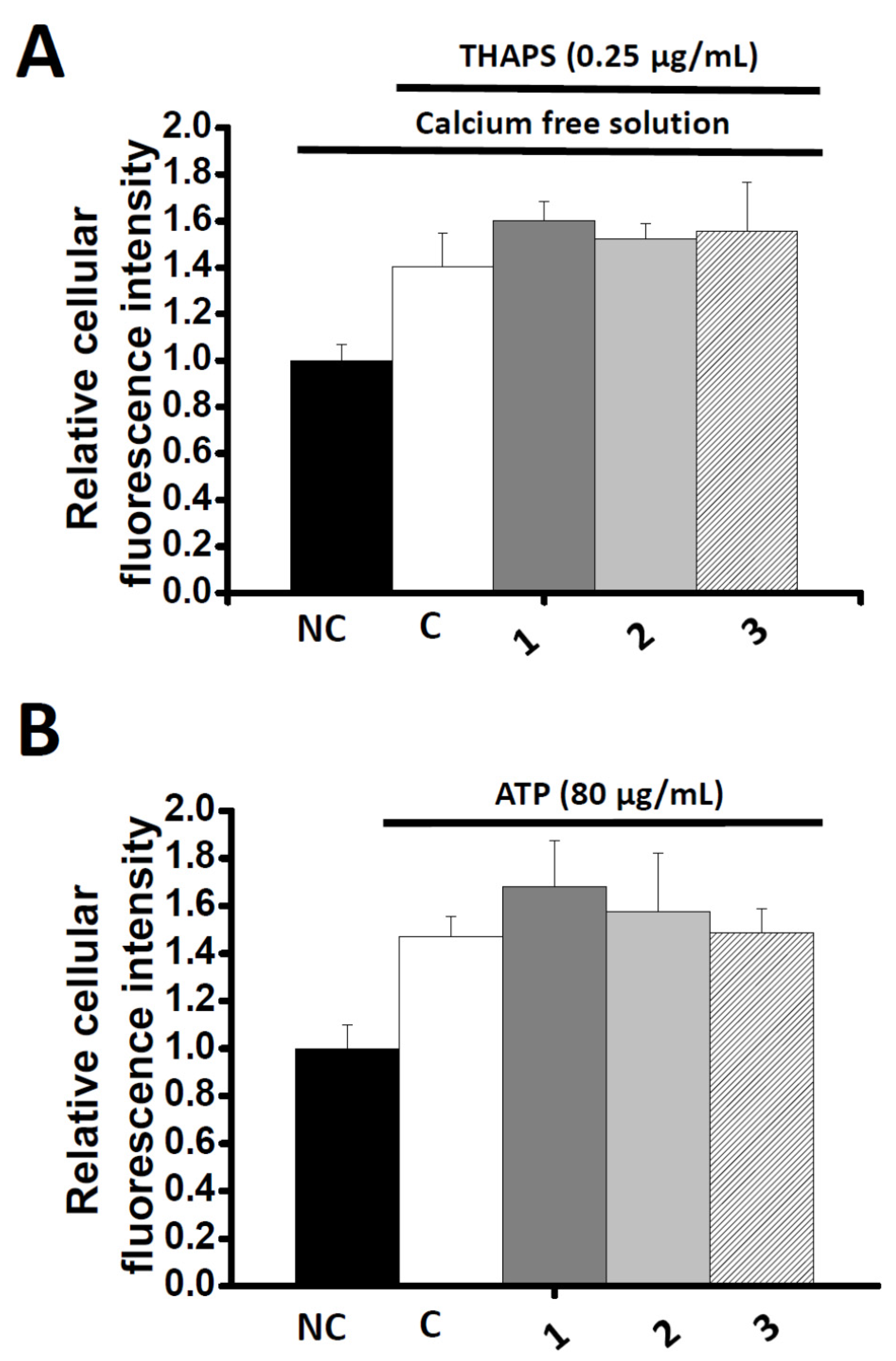
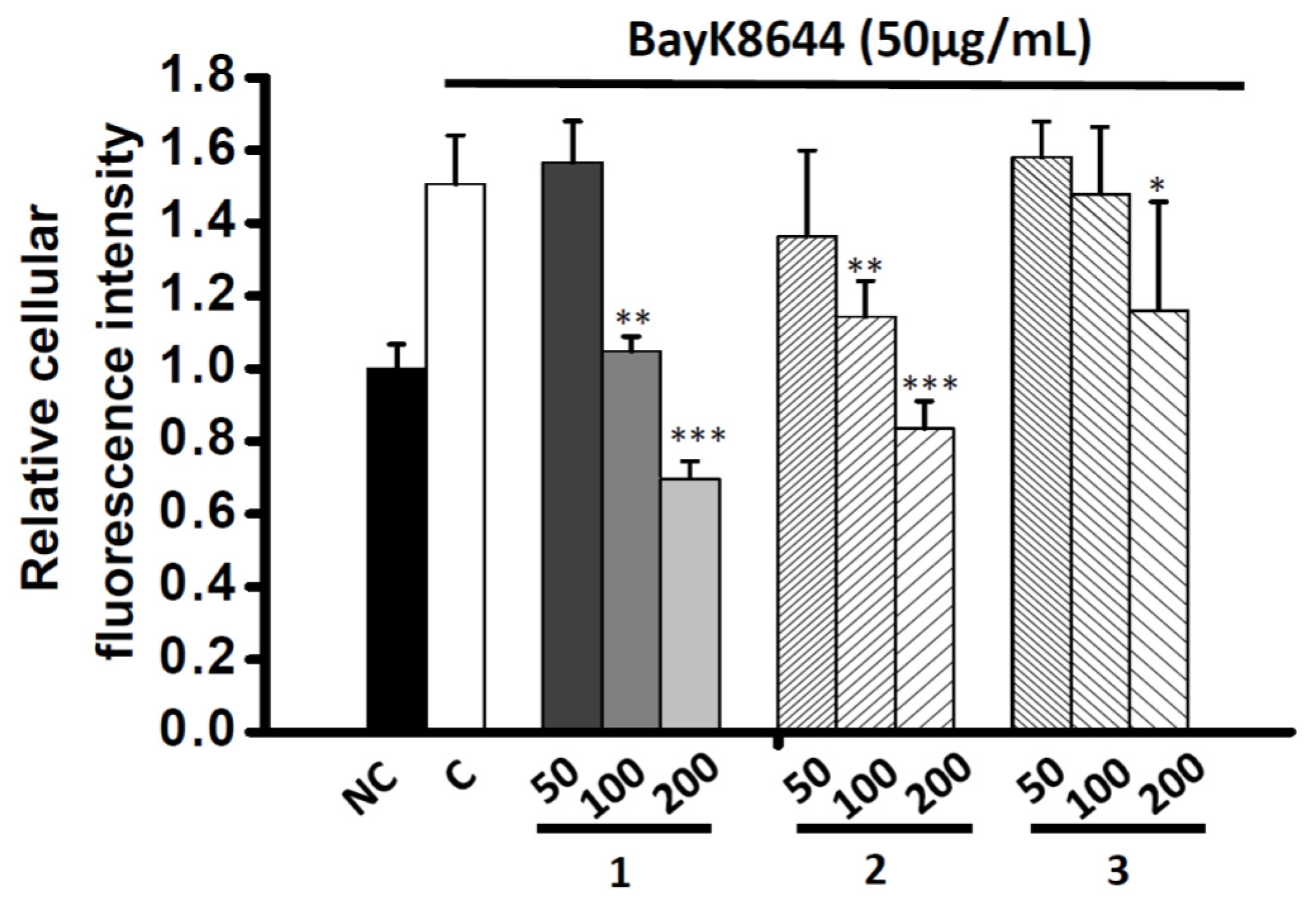
2.4. Effects of Co-administration Vasodilation Candidates with Different Ca2+-elevation Agents
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Preparation of Pine Needle Extract
4.3. LC/MS/MS Analysis
4.4. Intracellular Ca2+ Imaging
4.5. Isolation of Vasodilatation Candidates from Pine Needle Extract
4.6. Spectroscopic and Spectrometric Studies of Purified Compounds
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, K.Y.; Chung, H.J. Flavor compounds of pine sprout tea and pine needle tea. J. Agric. Food Chem. 2000, 48, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Hsieh, C.W.; Ko, W.C. Detection limit of molasses spirits mixed in rice spirits using the SNIF-NMR method. J. Food Drug Anal. 2014, 22, 197–201. [Google Scholar] [CrossRef]
- Liu, G.Y.; Wang, Z.W. Pinus morrisonicola Hayata extracts inhibit cell proliferation and promote apoptosis of human promyelocytic HL-60 leukemia cells. Eur. J. Cancer 2014, 50, S44. [Google Scholar] [CrossRef]
- Liao, C.L.; Chen, C.M.; Chang, Y.Z.; Liu, G.Y.; Hung, H.C.; Hsieh, T.Y.; Lin, C.L. Pine (Pinus morrisonicola Hayata) needle extracts sensitize GBM8901 human glioblastoma cells to temozolomide by downregulating autophagy and O(6)-methylguanine-DNA methyltransferase expression. J. Agric. Food Chem. 2014, 62, 10458–10467. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D.; Huang, D.W.; Hsu, C.L.; Fu, T.Y.C. Protective effect of pine (Pinus morrisonicola Hay.) needle on LDL oxidation and its anti-inflammatory action by modulation of NOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2008, 46, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Triggle, D.J. Calcium channel antagonists: Clinical uses-past, present and future. Biochem. Pharmacol. 2007, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Swanson, T.M. Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol. Pharmacol. 2015, 88, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi-Bensouda, L.; Klungel, O.; Kurz, X.; de Groot, M.C.; Maciel Afonso, A.S.; de Bruin, M.L.; Reynolds, R.; Rossignol, M. Calcium channel blockers and cancer: A risk analysis using the UK Clinical Practice Research Datalink (CPRD). BMJ Open 2016, 6, e009147. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Schiffrin, E.L.; White, W.B.; Mann, S.; Lindholm, L.H.; Kenerson, J.G.; Flack, J.M.; Carter, B.L.; Materson, B.J.; Ram, C.V.S.; et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American society of hypertension and the international society of hypertension. J. Clin. Hypertens. 2014, 16, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, J.; Tan, F.; Zhou, S.; Würthwein, G.; Rohdewald, P. Pycnogenol®, French maritime pine bark extract, improves endothelial function of hypertensive patients. Life Sci. 2004, 74, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.S.; Lim, D.Y. Pine needle extracts inhibit contractile responses of the isolated rat aortic strips. Nat. Prod. Sci. 2010, 16, 123–132. [Google Scholar]
- Jung, M.J.; Chung, H.Y.; Choi, J.H.; Choi, J.S. Antioxidant principles from the needles of red pine, Pinus densiflora. Phytother. Res. 2003, 17, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Seo, Y.H.; Cheong, H.S.; Lim, D.Y. Inhibitory effects of self-fermented pine needle extract on catecholamine release in the rat adrenal medulla. Nat. Prod. Sci. 2013, 19, 36–48. [Google Scholar]
- Moutzouri, E.; Florentin, M.; Elisaf, M.S.; Mikhailidis, D.P.; Liberopoulos, E.L. Aliskiren, a direct renin inhibitor, in clinical practice: A new approach in the treatment of hypertension. Curr. Vasc. Pharmacol. 2010, 8, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Bakris, G.L. Resistant hypertension: An overview of evaluation and treatment. J. Am. Coll. Cardiol. 2008, 52, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.; Eyles, A.; Chorbadjian, R.A.; Riedl, K.; Schwartz, S.; Hansen, R.; Cipollini, D.; Herms, D.A.; Bonello, P. Differential effects of nutrient availability on the secondary metabolism of Austrian pine (Pinus nigra) phloem and resistance to Diplodia pinea. For. Pathol. 2011, 41, 52–58. [Google Scholar] [CrossRef]
- Benayad, Z.; Gomez-Cordoves, C.; Es-Safi, N.E. Characterization of flavonoid glycosides from Fenugreek (Trigonella foenum-graecum) crude seeds by HPLC-DAD-ESI/MS analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Hamalainen, M.; Nieminen, R.; Klika, K.D.; Loponen, J.; Ovcharenko, V.V.; Moilanen, E.; Pohlaja, K. Phenolic extractives from the bark of Pinus sylvestris L. and their effects on inflammatory mediators nitric oxide and prostaglandin E-2. J. Agric. Food Chem. 2004, 52, 7532–7540. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shen, Y.H.; He, Y.R.; Zhang, W.D. Chemical constituents and biological activities of Pinus species. Chem. Biodivers. 2013, 10, 2133–2160. [Google Scholar] [CrossRef] [PubMed]
- Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Flavonoids and other phenolic compounds in needles of Pinus peuce and Other pine species from the Macedonian Flora. Nat. Prod. Commun. 2015, 10, 987–990. [Google Scholar] [PubMed]
- Nakanishi, T.; Iida, N.; Inatomi, Y.; Murata, H.; Inada, A.; Murata, J.; Lang, F.A.; Iinuma, M.; Tanaka, T. Neolignan and flavonoid glycosides in Juniperus communis var. depressa. Phytochemistry 2004, 65, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Nasr, C.; Haag-Berrurier, M.; Lobsterin-Guth, A.; Anton, R. Kaempferol coumaroyl glucorhamnoside from Ginko biloba. Phytochemistry 1986, 25, 770–771. [Google Scholar] [CrossRef]
- Fitzpatrick, D.F.; Bing, B.; Rohdewald, P. Endothelium-dependent vascular effects of Pycnogenol. J. Cardiovasc. Pharmacol. 1998, 32, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Junior, J.C.; Conserva, L.M.; Lyra Lemos, R.P.; de Omena-Neta, G.C.; Cavalcante-Neto, A.; Barreto, E. Isolation of a dihydrobenzofuran lignan, icariside E4, with an antinociceptive effect from Tabebuia roseo-alba (Ridley) Sandwith (Bignoniaceae) bark. Arch. Pharm. Res. 2015, 38, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Kim, J.H.; Choi, S.E.; Park, K.H.; Lee, M.W. Inhibitory effects of phenolic compounds from needles of Pinus densiflora on nitric oxide and PGE2 production. Arch. Pharm. Res. 2010, 33, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sánchez, M.A. Vasodilator compounds derived from plants and their mechanisms of action. Molecules 2013, 18, 5814–5857. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, K.; Hidaka, T.; Nakamura, S.; Umemura, T.; Jitsuiki, D.; Soga, J.; Goto, C.; Chayama, K.; Yoshizumi, M.; Higashi, Y. Pycnogenol®, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans. Hypertens. Res. 2007, 30, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, J.; Tan, F.; Zhou, S.; Würthwein, G.; Rohdewald, P. Antidiabetic effect of Pycnogenol French maritime pine bark extract in patients with diabetes type II. Life Sci. 2004, 75, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Rohdewald, P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002, 40, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Chovanova, Z.; Muchova, J.; Sumegová, K.; Liptáková, A.; Duracková, Z.; Högger, P. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol). Biomed. Pharmacother. 2006, 60, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [PubMed]
- Seo, M.J.; Kang, B.W.; Jeong, Y.K. Identification of a neolignan glycoside from the pine tree, Pinus densiflora showed antithrombotic activity. Life Sci. 2014, 24, 873–879. [Google Scholar] [CrossRef]
- Lo, Y.H.; Chen, Y.J.; Chang, C.I.; Lin, Y.W.; Chen, C.Y.; Lee, M.R.; Lee, V.S.Y.; Tzen, J.T.C. Teaghrelins, unique acylated flavonoid tetraglycosides in Chin-shin oolong tea, are putative oral agonists of the ghrelin receptor. J. Agric. Food Chem. 2014, 62, 5085–5091. [Google Scholar] [CrossRef] [PubMed]
- Campos-Toimil, M.; Elies, J.; Orallo, F. Trans- and cis-resveratrol increase cytoplasmic calcium levels in A7r5 vascular smooth muscle cells. Mol. Nutr. Food Res. 2005, 49, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Grabner, M.; Mitterdorfer, J.; Hering, S.; Sinnegger, M.J.; Glossmann, H. Structural basis of drug binding to L Ca2+ channels. Trends Pharmacol. Sci. 1998, 19, 108–115. [Google Scholar] [CrossRef]
- Cuíñas, A.; García-Morales, V.; Viña, D.; Gil-Longo, J.; Campos-Toimil, M. Activation of PKA and Epac proteins by cyclic AMP depletes intracellular calcium stores and reduces calcium availability for vasoconstriction. Life Sci. 2016, 155, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.H.; Chung, T.Y.; Li, F.Y.; Chen, H.A.; Tzen, J.T.C. Enhancing the potency of lithospermate B for inhibiting Na+/K+-ATPase activity by forming transition metal ion complexes. Acta Pharmacol. Sin. 2013, 34, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, J.M.; Ibarra, C.; Estrada, M.; Chiong, M.; Soto, D.; Parra, V.; Diaz-Araya, G.; Jaimovich, E.; Lavandero, S. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology 2006, 147, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the purified compounds 1–3 are available from the authors. |
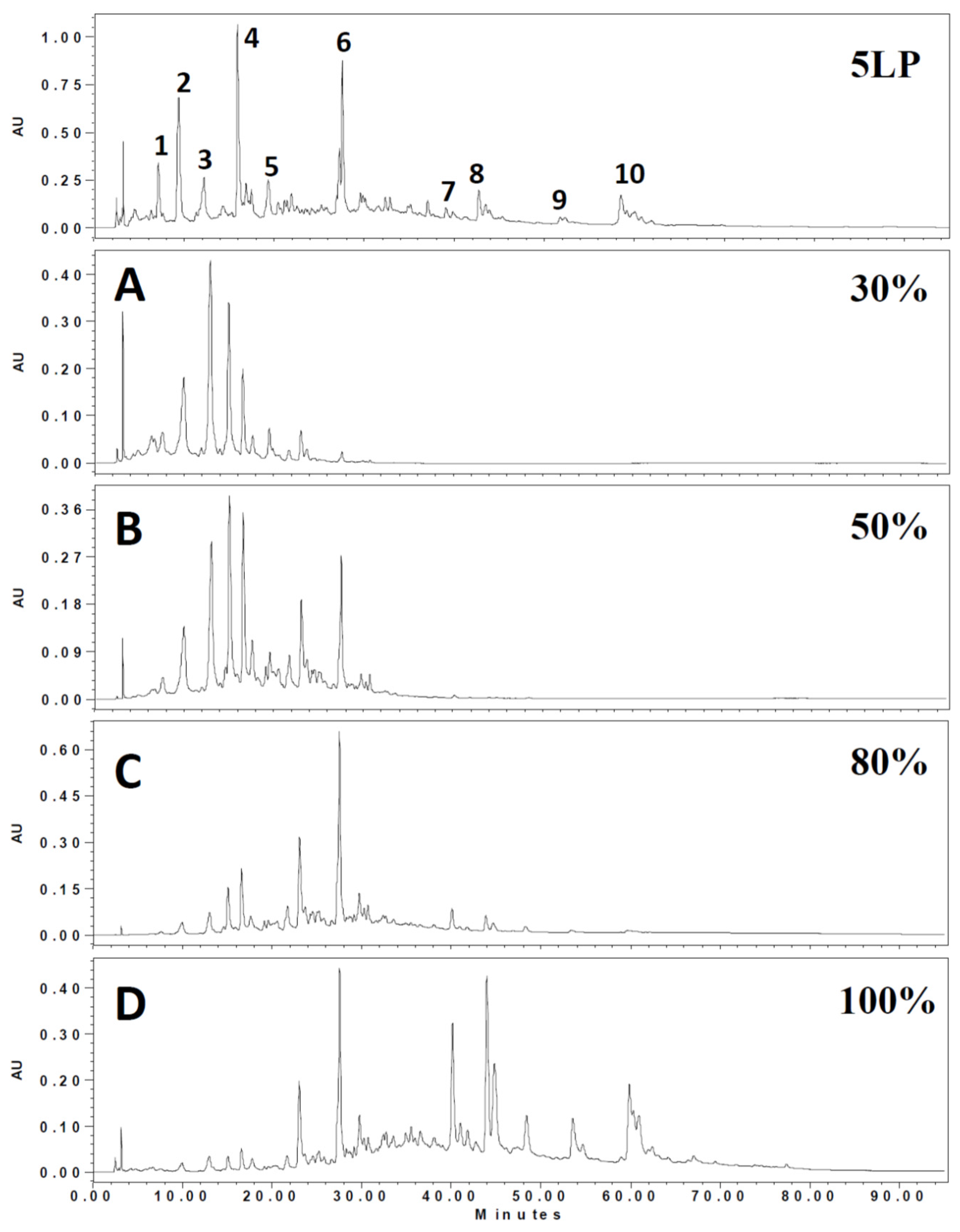

| Peak No. | RT (min) | λmax (nm) | [M – H]− (m/z) | MS2 (m/z) | Tentative Identification | Ref |
|---|---|---|---|---|---|---|
| 1 | 21.4 | 294 | 651 | 489,325,163 | Flavone-diglucoside | [19] |
| 2 | 22.2 | 316 | 647 | 485,383,323 | unknown | |
| 3 | 24.5 | 280 | 289 | 245,205,203,137 | Catechin | [20] |
| 4 | 30.0 | 313 | 671 | 323,119 | Dicaffeoyl-protocatechuic acid glucoside | [21] |
| 5 | 34.1 | 281 | 509 | 491,361,313 | Lignan glucoside | [22] |
| 6 | 40.0 | 281 | 507 | 491,359,341,329 | Icariside | [23] |
| 7 | 67.5 | 267,314 | 593 | 447,285 | Kaempferol coumaroyl-glucoside | [24] |
| 8 | 67.8 | 267,314 | 609 | 433,300 | Quercetin coumaroyl-rhamnose | [24] |
| 9 | 75.2 | 268,316 | 563 | 417,284 | Kaempferol coumaroyl-rhamnose | [24] |
| 10 | 76.1 | 267,314 | 739 | 593,485,285 | Kaempferol coumaroyl-glucose-rhamnoside | [24] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-H.; Li, Y.-C.; Lin, N.-H.; Kuo, P.-C.; Tzen, J.T.C. Characterization of Vasorelaxant Principles from the Needles of Pinus morrisonicola Hayata. Molecules 2018, 23, 86. https://doi.org/10.3390/molecules23010086
Chen G-H, Li Y-C, Lin N-H, Kuo P-C, Tzen JTC. Characterization of Vasorelaxant Principles from the Needles of Pinus morrisonicola Hayata. Molecules. 2018; 23(1):86. https://doi.org/10.3390/molecules23010086
Chicago/Turabian StyleChen, Guan-Heng, Yue-Chiun Li, Nan-Hei Lin, Ping-Chung Kuo, and Jason T. C. Tzen. 2018. "Characterization of Vasorelaxant Principles from the Needles of Pinus morrisonicola Hayata" Molecules 23, no. 1: 86. https://doi.org/10.3390/molecules23010086





