Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides
Abstract
:1. Introduction
2. Results and Discussion
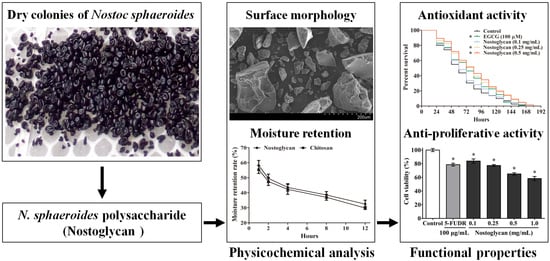
2.1. Physicochemical Characterization of Nostoglycan
2.2. Increase of Survival Rate and Reduction of ROS Levels by Nostoglycan in C. elegans under Oxidative Stress
2.3. Reduction of Protein Carbonyl and Malonaldehyde Contents and Upregulation of Antioxidant Enzyme Activities by Nostoglycan in C. elegans under Oxidative Stress
2.4. Inhibition of Proliferation of Human Tumor Cells by Nostoglycan
2.5. Induction of Apoptosis through Activation of Capspase-3 in Tumor Cells by Nostoglycan
3. Materials and Methods
3.1. Preparation of Polysaccharide
3.2. Determination of Carbohydrate Content and Analysis of Monosaccharide Composition
3.3. FTIR Spectroscopy Analysis
3.4. Congo Red Binding Assay
3.5. Surface Morphology Analysis
3.6. Assessment of Moisture Absorption and Retention
3.7. Determination of Viscosity
3.8. Nematode Maintenance
3.9. Paraquat Survival Assay in C. elegans
3.10. Determination of ROS Level in C. elegans
3.11. Determination of Protein Carbonyl and MDA Contents and Antioxidant Enzyme Activities in C. elegans
3.12. Tumor Cell Lines
3.13. Cell Viability Assay
3.14. Flow Cytometry Analysis of Cell Apoptosis
3.15. Measurement of Caspase-3 Activity
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, Z.; Liu, Y.D.; Paulsen, B.S.; Klaveness, D. Studies on polysaccharides from three edible species of Nostoc (cyanobacteria) with different colony morphologies: Comparison of monosaccharide compositions and viscosities of polysaccharides from field colonies and suspension cultures. J. Phycol. 1998, 34, 962–968. [Google Scholar] [CrossRef]
- Brüll, L.P.; Huang, Z.; Thomas-Oates, J.E.; Paulsen, B.S.; Cohen, E.H.; Michaelsen, T.E. Studies of polysaccharides from three edible species of Nostoc (cyanobacteria) with different colony morphologies: Structural characterization and effect on the complement system of polysaccharides from N. commune. J. Phycol. 2000, 36, 871–881. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Hu, Q.; Lu, F.; Liu, G.X.; Hu, Z.Y. Colony development and physiological characterization of the edible blue-green alga, Nostoc sphaeroides (Nostocaceae, Cyanophyta). Prog. Nat. Sci. 2008, 18, 1475–1483. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Liu, Y.; Ai, S.; Qin, F.; Li, Z.; Zhang, H.; Huang, Z. Antioxidant and moisture-retention activities of the polysaccharide from Nostoc commune. Carbohydr. Polym. 2011, 83, 1821–1827. [Google Scholar] [CrossRef]
- Ku, C.S.; Pham, T.X.; Park, Y.; Kim, B.; Shin, M.S.; Kang, I.; Lee, J. Edible blue-green algae reduce the production of pro-inflammatory cytokines by inhibiting NF-κB pathway in macrophages and splenocytes. Biochim. Biophys. Acta 2013, 1830, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, B.; Park, Y.K.; Lee, J.Y. Effects of long-term supplementation of blue-green algae on lipid metabolism in C57BL/6J mice. J. Nutr. Health Food Sci. 2014, 1, 6. [Google Scholar] [CrossRef]
- Ku, C.S.; Kim, B.; Pham, T.X.; Yang, Y.; Wegner, C.J.; Park, Y.K.; Balunas, M.; Lee, J.Y. Blue-green algae inhibit the development of atherosclerotic lesions in apolipoprotein E knockout mice. J. Med. Food 2015, 18, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kong, Y.H.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Höll, M.; Koziel, R.; Schäfer, G.; Pircher, H.; Pauck, A.; Hermann, M.; Klocker, H.; Jansen-Dürr, P.; Sampson, N. ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol. Carcinog. 2016, 55, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and oxidative stress: The molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm. 2015, 2015, 105828. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Shebis, Y.; Iluz, D.; Kinel-Tahan, Y.; Dubinsky, Z.; Yehoshua, Y. Natural antioxidants: Function and sources. Food Nutr. Sci. 2013, 4, 643–649. [Google Scholar] [CrossRef]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Potts, M. Desiccation tolerance of procaryotes. Microbiol. Rev. 1994, 58, 755–805. [Google Scholar] [PubMed]
- Wang, G.; Hu, C.; Li, D.; Zhang, D.; Li, X.; Chen, K.; Liu, Y. The response of antioxidant systems in Nostoc sphaeroides against UV-B radiation and the protective effects of exogenous antioxidants. Adv. Space Res. 2007, 39, 1034–1042. [Google Scholar] [CrossRef]
- Tang, J.; Hu, Z.Y.; Chen, X.W. Free radical scavenging and antioxidant enzymes activation of polysaccharide extract from Nostoc sphaeroides. Am. J. Chin. Med. 2007, 35, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Newland, P.; Bingham, B.; Tarelli, E.; Thomas, A.H. High-performance gel permeation chromatography of meningococcal polysaccharides. J. Chromatogr. 1989, 483, 406–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Semedo, M.C.; Karmali, A.; Fonseca, L. A high throughput colorimetric assay of β-1,3-d-glucans by Congo red dye. J. Microbiol. Methods 2015, 109, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhu, P.; Wang, M.; Ma, S.; Wei, Z. Optimization of water-soluble polysaccharides from stem lettuce by response surface methodology and study on its characterization and bioactivities. Int. J. Biol. Macromol. 2017, 105, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podestà, A.; Milani, P. The effect of surface nanometre-scale morphology on protein adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.R.; Yu, H.F.; Lin, Y.X.; Dai, Y.J. Characterization of extracellular polysaccharides from Nostoc flagelliforme cells in liquid suspension culture. Biotechnol. Bioprocess Eng. 2007, 12, 271–275. [Google Scholar] [CrossRef]
- Nep, E.I.; Conway, B.R. Physicochemical characterization of grewia polysaccharide gum: Effect of drying method. Carbohydr. Polym. 2011, 84, 446–453. [Google Scholar] [CrossRef]
- Helm, R.F.; Huang, Z.; Edwards, D.; Leeson, H.; Peery, W.; Potts, M. Structural characterization of the released polysaccharide of desiccation-tolerant Nostoc commune DRH-1. J. Bacteriol. 2000, 182, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Hill, D.R.; Brittain, N.; Wright, D.J.; Taüber, U.; Marand, H.; Helm, R.F.; Potts, M. Unusual water flux in the extracellular polysaccharide of the cyanobacterium Nostoc commune. Appl. Environ. Microbiol. 2003, 69, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Or, D.; Phutane, S.; Dechesne, A. Extracellular polymeric substances affecting pore-scale hydrologic conditions for bacterial activity in unsaturated soils. Vadose Zone J. 2007, 6, 298–305. [Google Scholar] [CrossRef]
- Mo, K.; Cheng, C.; Zhuang, Y.; Chen, M.; Zhao, N. Conformational analysis of polysaccharide from Nostoc sphaeroides Kütz. based on atomic force microscope and rheological properties. Food Sci. 2017, 38, 49–54. [Google Scholar]
- Zhang, J.; Shi, R.; Li, H.; Xiang, Y.; Xiao, L.; Hu, M.; Ma, F.; Ma, C.W.; Huang, Z. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J. Ethnopharmacol. 2016, 192, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Y.; Qin, C.; Liang, M.; Mao, X.; Li, S.; Zou, Y.; Jia, W.; Li, H.; Ma, C.W.; et al. Bioactive peptides from Angelica sinensis protein hydrolyzate delay senescence in Caenorhabditis elegans through antioxidant activities. Oxid. Med. Cell. Longev. 2016, 2016, 8956981. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, J.; Li, H.; Wang, Q.; Xiao, L.; Weng, H.; Zhou, X.; Ma, C.W.; Ma, F.; Hu, M.; et al. Epimedium polysaccharide alleviates polyglutamine-induced neurotoxicity in Caenorhabditis elegans by reducing oxidative stress. Rejuvenation Res. 2017, 20, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.X.; Edwards, B.; Lee, S.; Finelli, M.J.; Davies, B.; Davies, K.E.; Oliver, P.L. Neuron-specific antioxidant OXR1 extends survival of a mouse model of amyotrophic lateral sclerosis. Brain 2015, 138, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E. Role of antioxidants in paraquat toxicity. Toxicology 2002, 180, 65–77. [Google Scholar] [CrossRef]
- Medina-Navarro, R.; Nieto-Aguilar, R.; Alvares-Aguilar, C. Protein conjugated with aldehydes derived from lipid peroxidation as an independent parameter of the carbonyl stress in the kidney damage. Lipids Health Dis. 2011, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Srivastava, S.; Singhal, S.S. Lipid peroxidation products in human health and disease 2014. Oxid. Med. Cell. Longev. 2014, 2014, 162414. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Xiang, Y.; Xiang, L.; Liu, Y.; He, X.; Zhou, X.; Liu, X.; Huang, Z. Extracts of Tsai Tai (Brassica chinensis): Enhanced antioxidant activity and anti-aging effects both in vitro and in Caenorhabditis elegans. Food Funct. 2016, 7, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Vistoli, G.; Stefek, M.; Chondrogianni, N.; Grune, T.; Sereikaite, J.; Sadowska-Bartosz, I.; Bartosz, G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013, 47 (Suppl. 1), 93–137. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, P.; García-Beltrán, O.; Tapia, V.; Muñoz, Y.; Cassels, B.K.; Núñez, M.T. Neuroprotective effect of a new 7,8-dihydroxycoumarin-based Fe2+/Cu2+ chelator in cell and animal models of Parkinson's disease. ACS Chem. Neurosci. 2017, 8, 178–185. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Paperi, R.; Sili, C. Heavy metal sorption by released polysaccharides and whole cultures of two exopolysaccharide-producing cyanobacteria. Biodegradation 2007, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortee, S.; Liu, Y.; Zhang, L.; Matthews, H.R.; Zhang, H.; Pan, N.; Cheng, C.R.; Guan, S.H.; Guo, D.A.; Huang, Z.; et al. Macromolecular and small-molecule modulation of intracellular Aβ42 aggregation and associated toxicity. Biochem. J. 2012, 442, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Li, H.; Fang, Z.; Lin, J.; Wang, S.; Xiao, L.; Yang, F.; Liu, X.; Zhang, J.; Huang, Z.; et al. Polysaccharides from Angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regen. Res. 2014, 9, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Lv, Z.; Qiao, X.; Li, X.; Li, Y.; Zhang, Y.; Chen, C. The decay of redox-stress response capacity is a substantive characteristic of aging: Revising the redox theory of aging. Redox Biol. 2017, 11, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Cheon, S.M.; Lee, M.H.; Kim, H.J.; Jeon, H.; Cha, D.S. Catalpol modulates lifespan via DAF-16/FOXO and SKN-1/Nrf2 activation in Caenorhabditis elegans. Evid. Based Complement. Alternat. Med. 2015, 2015, 524878. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515–549. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Xiong, S.; Zhang, H.; Li, N.; Zhou, S.; Liu, Y.; Huang, Z. Pilot-scale isolation of bioactive extracellular polymeric substances from cell-free media of mass microalgal cultures using tangential-flow ultrafiltration. Process Biochem. 2011, 46, 1104–1109. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ding, G.B.; Guo, S.; Li, Z.; Zhao, L.; Li, K.; Guo, X. Isolation and antitumor efficacy evaluation of a polysaccharide from Nostoc commune Vauch. Food Funct. 2015, 6, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.J.; Yu, J.; Ji, H.Y.; Zhang, H.C.; Zhang, Y.; Liu, H.P. Extraction of a novel cold-water-soluble polysaccharide from Astragalus membranaceus and its antitumor and immunological activities. Molecules 2017, 23, E62. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Liu, Y.D.; Song, L.R. Hormogonia mass differentiation from Nostoc sphaeroides Kütz. (cyanobacterium) and the comparison of structural characteristics between hormogonia and vegetative filaments. Phycol. Res. 2001, 49, 81–87. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments. Food Chem. 2016, 197, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, G.; Yu, Z.; Song, X.; Li, X.; Yang, Y.; Wang, L.; Liu, L.; Dai, J. Purification, characterization and antiglycation activity of a novel polysaccharide from black currant. Food Chem. 2016, 199, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Bo, S.; Cheng, R.; Jiang, L.; Yang, Y. Analysis of interfacial phenomena of aqueous solutions of polyethylene oxide and polyethylene glycol flowing in hydrophilic and hydrophobic capillary viscometers. J. Colloid Interface Sci. 2004, 276, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Yuan, Y.; Sun, Y.; Qin, Y.; Deng, Z.; Li, H. Protective effects of selenium, vitamin E, and purple carrot anthocyanins on d-galactose-induced oxidative damage in blood, liver, heart and kidney rats. Biol. Trace Elem. Res. 2016, 173, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Li, H.; Zhang, J.; Yang, F.; Huang, A.; Deng, J.; Liang, M.; Ma, F.; Hu, M.; Huang, Z. Salidroside protects Caenorhabditis elegans neurons from polyglutamine-mediated toxicity by reducing oxidative stress. Molecules 2014, 19, 7757–7769. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Jia, T.; Ai, W.; Duan, J.; Liu, Y.; Chen, J.; Liu, X.; Yang, F.; Tian, Y.; Huang, Z. Targeted delivery of doxorubicin through conjugation with EGF receptor-binding peptide overcomes drug resistance in human colon cancer cells. Br. J. Pharmacol. 2013, 168, 1719–1735. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Li, J.; Li, Y.; Chu, C.; Xie, G.; Qin, J.; Yang, M.; Zhuang, D.; Cui, L.; Zhang, H.; et al. N-acetylcysteine protects Chinese hamster ovary cells from oxidative injury and apoptosis induced by microcystin-LR. Int. J. Clin. Exp. Med. 2015, 8, 4911–4921. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |





| Treatment | Protein Carbonyl Content a | MDA Content b | SOD Activity c | CAT Activity d | GPx Activity c |
|---|---|---|---|---|---|
| Control | 0.88 ± 0.15 | 7.60 ± 0.54 | 49.02 ± 4.66 | 1.20 ± 0.02 | 15.59 ± 1.63 |
| Nostoglycan | 0.82 ± 0.12 | 4.94 ± 0.31 e | 46.81 ± 6.84 | 1.32 ± 0.02 e | 16.87 ± 1.50 |
| Paraquat | 1.71 ± 0.14 e | 10.39 ± 0.87 e | 69.97 ± 3.51 e | 1.08 ± 0.02 e | 15.09 ± 0.76 |
| Nostoglycan + Paraquat | 1.02 ± 0.18 f | 7.28 ± 0.46 f | 97.88 ± 6.72 f | 1.21 ± 0.02 f | 14.19 ± 1.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides. Molecules 2018, 23, 508. https://doi.org/10.3390/molecules23020508
Li H, Su L, Chen S, Zhao L, Wang H, Ding F, Chen H, Shi R, Wang Y, Huang Z. Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides. Molecules. 2018; 23(2):508. https://doi.org/10.3390/molecules23020508
Chicago/Turabian StyleLi, Haifeng, Linnan Su, Sheng Chen, Libin Zhao, Hongyu Wang, Fei Ding, Hong Chen, Ruona Shi, Yulan Wang, and Zebo Huang. 2018. "Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides" Molecules 23, no. 2: 508. https://doi.org/10.3390/molecules23020508