Structural Characterization of Lithium and Sodium Bulky Bis(silyl)amide Complexes
Abstract
:1. Introduction
2. Results
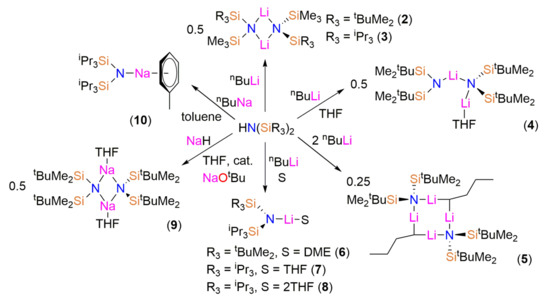
2.1. Synthesis
2.2. Spectroscopy
2.3. Structural Characterization
3. Discussion
4. Materials and Methods
General Information
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lappert, M.F.; Power, P.P.; Sanger, A.R.; Srivastava, R.C. Metal and Metalloid Amides; Ellis Horwood-Wiley: Chichester, UK, 1980. [Google Scholar]
- Lappert, M.F.; Protchenko, A.; Power, P.P.; Seeber, A. Metal Amide Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2008. [Google Scholar]
- Kays, D.L. Extremely bulky amide ligands in main group chemistry. Chem. Soc. Rev. 2016, 45, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, R.E.; Robertson, S.D. Synthetically Important Alkali-Metal Utility Amides: Lithium, Sodium and Potassium Hexamethyldisilazides, Diisopropylamides and Tetramethylpiperidides. Angew. Chem. Int. Ed. 2013, 52, 11470–11487. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.P. The role of the bis-trimethylsilylamido ligand, [N{SiMe3}2]–, in main group chemistry. Part 1: Structural chemistry of the s-block elements. Coord. Chem. Rev. 2015, 297–298, 2–23. [Google Scholar] [CrossRef]
- Coles, M.P. The role of the bis-trimethylsilylamido ligand, [N{SiMe3}2]–, in main group chemistry. Part 2 Structural chemistry of the metallic p-block elements. Coord. Chem. Rev. 2015, 297–298, 24–39. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Mills, D.P. Silylamides: Towards a half-century of stabilising remarkable f-element chemistry. Organomet. Chem. 2017, 41, 123–156. [Google Scholar]
- Mootz, D.; Zinnius, A.; Böttcher, B. Association of Bis(trimethylsilyl)amidolithium and Methyl(trimethylsilanolato)beryllium in the Solid State. Angew. Chem. Int. Ed. 1969, 8, 378–379. [Google Scholar] [CrossRef]
- Rogers, R.D.; Atwood, J.L.; Grüning, R. The crystal structure of N-lithiohexamethyldisilazane, [LiN(SiMe3)2]3. J. Organomet. Chem. 1978, 157, 229–237. [Google Scholar] [CrossRef]
- Grüning, R.; Atwood, J.L. The crystal structure of N-sodiohexamethyldisilazane, Na[N{Si(CH3)3}2]. J. Organomet. Chem. 1977, 137, 101–111. [Google Scholar] [CrossRef]
- Tesh, K.F.; Hanusa, T.P.; Huffman, J.C. Ion Pairing in [Bis(trimethylsilyl)amido]potassium: X-ray Crystal Structure of Unsolvated [KN(SiMe3)2]2. Inorg. Chem. 1990, 29, 1584–1586. [Google Scholar] [CrossRef]
- Bowser, J.R.; Neilson, R.H.; Wells, R.L. Preparation and Thermal Decomposition Reactions of Some tert-Butyldimethylsilyl-Substituted Aminoboranes. Inorg. Chem. 1978, 17, 1882–1886. [Google Scholar] [CrossRef]
- Tang, Y.; Zakharov, L.N.; Kassel, W.S.; Rheingold, A.L.; Kemp, R.A. Synthesis and structural characterization of solvated calcium amides containing bulky silylamide ligands. Inorg. Chim. Acta 2005, 358, 2014–2022. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Joslin, K.C.; Lockyer, S.J.; Formanuik, A.; Morris, G.A.; Ortu, F.; Vitorica-Yrezabal, I.J.; Mills, D.P. Homoleptic Trigonal Planar Lanthanide Complexes Stabilized by Superbulky Silylamide Ligands. Organometallics 2015, 34, 2314–2325. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Tuna, F.; McInnes, E.J.L.; Liddle, S.T.; McMaster, J.; Vitorica-Yrezabal, I.J.; Mills, D.P. [UIII{N(SiMe2tBu)2}3]: A Structurally Authenticated Trigonal Planar Actinide Complex. Chem. Eur. J. 2014, 20, 14579–14583. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Goodwin, C.A.P.; Mills, D.P.; Winpenny, R.E.P. The first near-linear bis(amide) f-block complex: A blueprint for a high temperature single molecule magnet. Chem. Commun. 2015, 51, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P.; Chilton, N.F.; Vettese, G.F.; Moreno Pineda, E.; Crowe, I.F.; Ziller, J.W.; Winpenny, R.E.P.; Evans, W.J.; Mills, D.P. Physicochemical properties of near-linear Ln(II) bis-silylamide complexes (Ln = Sm, Eu, Tm, Yb). Inorg. Chem. 2016, 55, 10057–10067. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P.; Smith, A.; Ortu, F.; Vitorica-Yrezabal, I.J.; Mills, D.P. Salt metathesis versus protonolysis routes for the synthesis of silylamide Hauser base (R2NMgX; X = halogen) and amido-Grignard (R2NMgR) complexes. Dalton Trans. 2016, 45, 6004–6014. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P.; Chilton, N.F.; Natrajan, L.S.; Boulon, M.-E.; Ziller, J.W.; Evans, W.J.; Mills, D.P. An Investigation into the Effects of a Trigonal Planar Ligand Field on the Electronic Properties of Lanthanide(II) Tris(silylamide) Complexes (Ln = Sm, Eu, Tm, Yb). Inorg. Chem. 2017, 56, 5959–5970. [Google Scholar] [CrossRef] [PubMed]
- Ortu, F.; Gregson, M.; Wooles, A.J.; Mills, D.P.; Liddle, S.T. Yttrium methanide and methanediide bis(silylamides). Organometallics 2017, 36, 4584–4590. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Tuna, F.; McInnes, E.J.L.; Mills, D.P. Exploring Synthetic Routes to Heteroleptic UIII, UIV and ThIV Bulky Bis(silyl)amide Complexes. Eur. J. Inorg. Chem. 2018. [Google Scholar] [CrossRef]
- Leng, J.-D.; Goodwin, C.A.P.; Vitorica-Yrezabal, I.J.; Mills, D.P. Salt metathesis routes to homoleptic near-linear Mg(II) and Ca(II) bulky bis(silyl)amide complexes. Dalton Trans. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mansell, S.M.; Fernandez Perandones, B.; Arnold, P.L. New UIII and UIV silylamides and an improved synthesis of NaN(SiMe2R)2 (R = Me, Ph). J. Organomet. Chem. 2010, 695, 2814–2821. [Google Scholar] [CrossRef]
- Schade, C.; Bauer, W.; Von Ragué Schleyer, P. n-Butylsodium: The preparation, properties and NMR spectra of a hydrocarbon- and tetrahydrofuran-soluble reagent. J. Organomet. Chem. 1985, 295, C25–C28. [Google Scholar] [CrossRef]
- Robiette, A.G.; Sheldrick, G.M.; Sheldrick, W.S.; Beagley, B.; Cruickshank, D.W.J.; Monaghan, J.J.; Aylett, B.J.; Ellis, I.A. The conformation of three disilanes. Chem. Commun. 1968, 909–910. [Google Scholar]
- Bartlett, R.A.; Power, P.P. A Silicon-Nitrogen Analogue of the [PPN]+ Cation: Synthesis and Structural Characterization of the [Ph3SiNSiPh3]– Ion. J. Am. Chem. Soc. 1987, 109, 6509–6510. [Google Scholar] [CrossRef]
- Kimura, B.Y.; Brown, T.L. Solvent effects on the aggregation of lithium bis(trimethylsilyl)amide. J. Organomet. Chem. 1971, 26, 57–67. [Google Scholar] [CrossRef]
- Ortu, F.; Moxey, G.J.; Blake, A.J.; Lewis, W.; Kays, D.L. Alkaline Earth Complexes of Silylated Aminopyridinato Ligands: Homoleptic Compounds and Heterobimetallic Coordination Polymers. Inorg. Chem. 2013, 52, 12429–12439. [Google Scholar] [CrossRef] [PubMed]
- Francos, J.; Fleming, B.J.; Garcia-Álvarez, P.; Kennedy, A.R.; Reilly, K.; Robertson, G.M.; Robertson, S.D.; O’Hara, C.T. Complexity in seemingly simple sodium magnesiate systems. Dalton Trans. 2014, 43, 14424–14431. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, A.J.; Armstrong, D.R.; Conway, B.; Fleming, B.J.; Klett, J.; Kennedy, A.R.; Mulvey, R.E.; Robertson, S.D.; O’Hara, C.T. Pre-inverse-crowns: Synthetic, structural and reactivity studies of alkali metal magnesiates primed for inverse crown formation. Chem. Sci. 2014, 5, 771–781. [Google Scholar] [CrossRef]
- Henderson, K.W.; Dorigo, A.E.; Liu, Q.-Y.; Williard, P.G. Effect of Polydentate Donor Molecules on Lithium Hexamethyldisilazide Aggregation: An X-ray Crystallographic and a Combination Semiempirical PM3/Single Point ab Initio Theoretical Study. J. Am. Chem. Soc. 1997, 119, 11855–11863. [Google Scholar] [CrossRef]
- Engelhardt, L.M.; Jolly, B.S.; Junk, P.C.; Raston, C.L.; Skelton, B.W.; White, A.H. Highly Hindered Amido-Lithium and Amido-Magnesium Complexes. Crystal-Structures of [Li(μ-N(SiMe3)2)(Tetrahydrofuran)]2 and [MgBus(μ-N(SiMe3)2)]2. Aust. J. Chem. 1986, 39, 1337–1345. [Google Scholar] [CrossRef]
- Mack, H.; Frenzen, G.; Bendikov, M.; Eisen, M.S. Thermal decomposition of [Me3SiNCH2CH2NSiMe3]·Li2(THF)2 to [LiN(SiMe3)2·THF]2 via a 1,4-trimethylsilyl shift. J. Organomet. Chem. 1997, 549, 39–43. [Google Scholar] [CrossRef]
- Karl, M.; Seybert, G.; Massa, W.; Harms, K.; Agarwal, S.; Maleika, R.; Stelter, W.; Greiner, A.; Heitz, W.; Neumüller, B.; et al. Amidometallate von Seltenerdelementen. Synthese und Kristallstrukturen von [Na(12-Krone-4)2][M{N(SiMe3)2}3(OSiMe3)] (M = Sm, Yb), [Na(THF)3Sm{N(SiMe3)2}3(C≡C–Ph)], [Na(THF)6][Lu2(μ-NH2)(μ-NSiMe3){N(SiMe3)2}4] sowie von [NaN(SiMe3)2(THF)]2. Anwendungen der Seltenerdkomplexe als Polymerisationskatalysatoren. Z. Anorg. Allg. Chem. 1999, 625, 1301–1309. [Google Scholar]
Sample Availability: Samples of the compounds 2–4, 7, 9 and 10 are available from the authors. |











| Distance (Å)/Angle (°) | 1 | 2 | 3 | 4 | 5 |
| N(1)–Si(1) | 1.7348(12) | 1.701(2) | 1.711(2) | 1.712(4) | 1.714(4) |
| N(1)–Si(2) | 1.7370(12) | 1.704(2) | 1.707(2) | 1.728(4) | 1.713(5) |
| Li(1)–N(1) | - | 2.015(5) | 2.022(4) | 1.974(10) | 1.986(9) |
| Li(1)–N(2) | - | 1.986(5) | 2.032(4) | - | - |
| Li(2)–N(1) | - | 1.982(5) | 2.036(4) | 2.026(8) | 1.946(12) |
| Li(2)–N(2) | - | 2.023(5) | 2.030(4) | 1.915(9) | - |
| N(2)–Si(3) | - | 1.706(2) | 1.708(2) | 1.667(4) | - |
| N(2)–Si(4) | - | 1.701(2) | 1.705(2) | 1.679(3) | - |
| Li(1)–O(1) | - | - | - | 1.891(8) | - |
| Li(1)–C(13) | - | - | - | - | 2.037(9) |
| Li···C distances < 2.5 Å | - | 2.378(6) | 2.378(6) | - | - |
| - | 2.392(7) | 2.392(7) | - | - | |
| - | 2.393(7) | - | - | - | |
| - | 2.427(6) | - | - | - | |
| Si(1)–N(1)–Si(2) | 145.43(8) | 125.33(12) | 127.91(11) | 121.0(2) | 131.0(3) |
| Si(3)–N(2)–Si(4) | - | 125.29(13) | 127.87(11) | 144.2(2) | - |
| N(1)–Li(1)–N(2) | - | 109.1(2) | 110.1(2) | - | |
| N(1)–Li(2)–N(2) | - | 109.0(2) | 110.0(2) | 160.2(4) | - |
| Li(1)–N(1)–Li(2) | - | 71.0(2) | 69.8(2) | 97.6(3) | 95.7(5) |
| Li(1)–N(2)–Li(2) | - | 70.8(2) | 69.5(2) | - | - |
| N(1)–Li(1)–O(1) | - | - | - | 155.4(5) | - |
| N(1)–Li(1)–C(13) | - | - | - | - | 171.9(5) |
| N(1A)–Li(2A)–C(13) | - | - | - | - | 173.9(7) |
| Li(1)–C(13)–Li(2A) | - | - | - | - | 91.7(4) |
| Distance (Å)/Angle (°) | 6 | 7 | 8 | 9-THF | 10 |
| N(1)–Si(1) | 1.681(2) | 1.6761(13) | 1.686(2) | 1.697(3) | 1.6709(12) |
| N(1)–Si(2) | 1.679(5) | 1.6805(13) | 1.681(2) | 1.702(3) | 1.6710(13) |
| M(1)–N(1) | 1.894(13) | 1.893(4) | 1.953(5) | 2.490(2) | 2.2608(14) |
| M(1)–N(2) | - | - | - | - | - |
| M(2)–N(1) | - | - | - | - | - |
| M(2)–N(2) | - | - | - | - | - |
| M(1)–O(1) | 1.984(5) | 1.866(3) | 1.987(5) | 2.328(3) | - |
| M(1)–O(2) | 1.958(3) | - | 1.957(5) | - | - |
| Na(1)···C(19) | - | - | - | - | 2.912(2) |
| Na(1)···C(20) | - | - | - | - | 2.815(2) |
| Na(1)···C(21) | - | - | - | - | 2.801(2) |
| Na(1)···C(22) | - | - | - | - | 2.870(2) |
| Na(1)···C(23) | - | - | - | - | 2.935(2) |
| Na(1)···C(24) | - | - | - | - | 2.956(2) |
| Na(1)···Phcentroid | - | - | - | - | 2.527(2) |
| Si(1)–N(1)–Si(2) | 135.08(13) | 142.07(8) | 143.06(13) | 124.18(14) | 144.09(8) |
| N(1)–Na(1)–N(1A) | - | - | - | 108.47(7) | - |
| Na(1)–N(1)–Na(1A) | - | - | - | 71.53(7) | - |
| N(1)–M(1)–O(1) | 143.4(3) | 168.7(2) | 132.5(3) | 126.51(7) | - |
| N(1)–M(1)–O(2) | 133.6(2) | - | 130.7(3) | - | - |
| O(1)–M(1)–O(2) | 82.8(2) | - | 96.6(2) | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholas, H.M.; Goodwin, C.A.P.; Kragskow, J.G.C.; Lockyer, S.J.; Mills, D.P. Structural Characterization of Lithium and Sodium Bulky Bis(silyl)amide Complexes. Molecules 2018, 23, 1138. https://doi.org/10.3390/molecules23051138
Nicholas HM, Goodwin CAP, Kragskow JGC, Lockyer SJ, Mills DP. Structural Characterization of Lithium and Sodium Bulky Bis(silyl)amide Complexes. Molecules. 2018; 23(5):1138. https://doi.org/10.3390/molecules23051138
Chicago/Turabian StyleNicholas, Hannah M., Conrad A. P. Goodwin, Jon G. C. Kragskow, Selena J. Lockyer, and David P. Mills. 2018. "Structural Characterization of Lithium and Sodium Bulky Bis(silyl)amide Complexes" Molecules 23, no. 5: 1138. https://doi.org/10.3390/molecules23051138