Phytochemical Screening of Quaking Aspen (Populus tremuloides) Extracts by UPLC-QTOF-MS and Evaluation of their Antimicrobial Activity
Abstract
:1. Introduction
2. Results
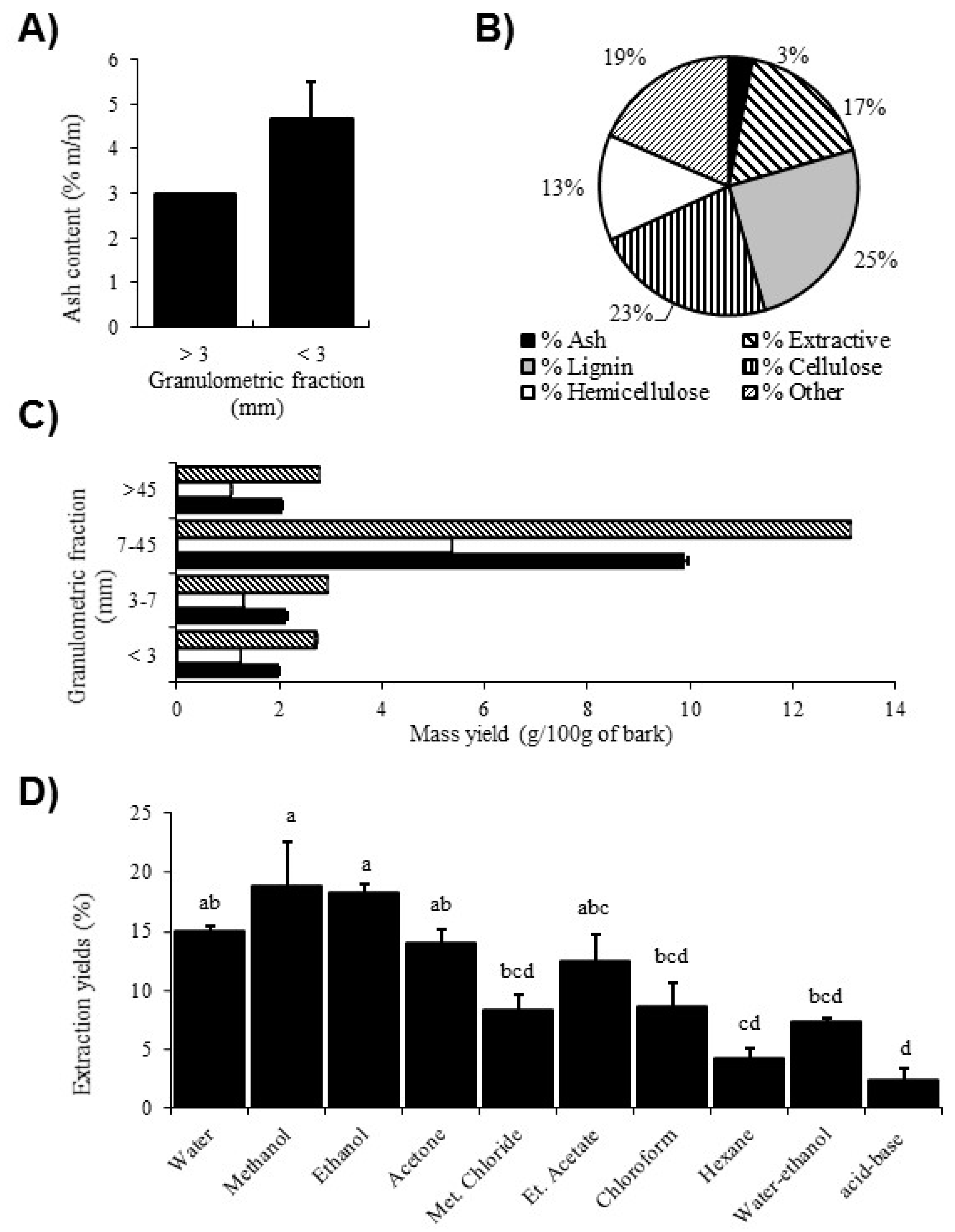
2.1. Determination of Bark Composition and Extractive Yield per Fraction Size
2.2. Determination of Extractive Yield According to Molecule Polarity
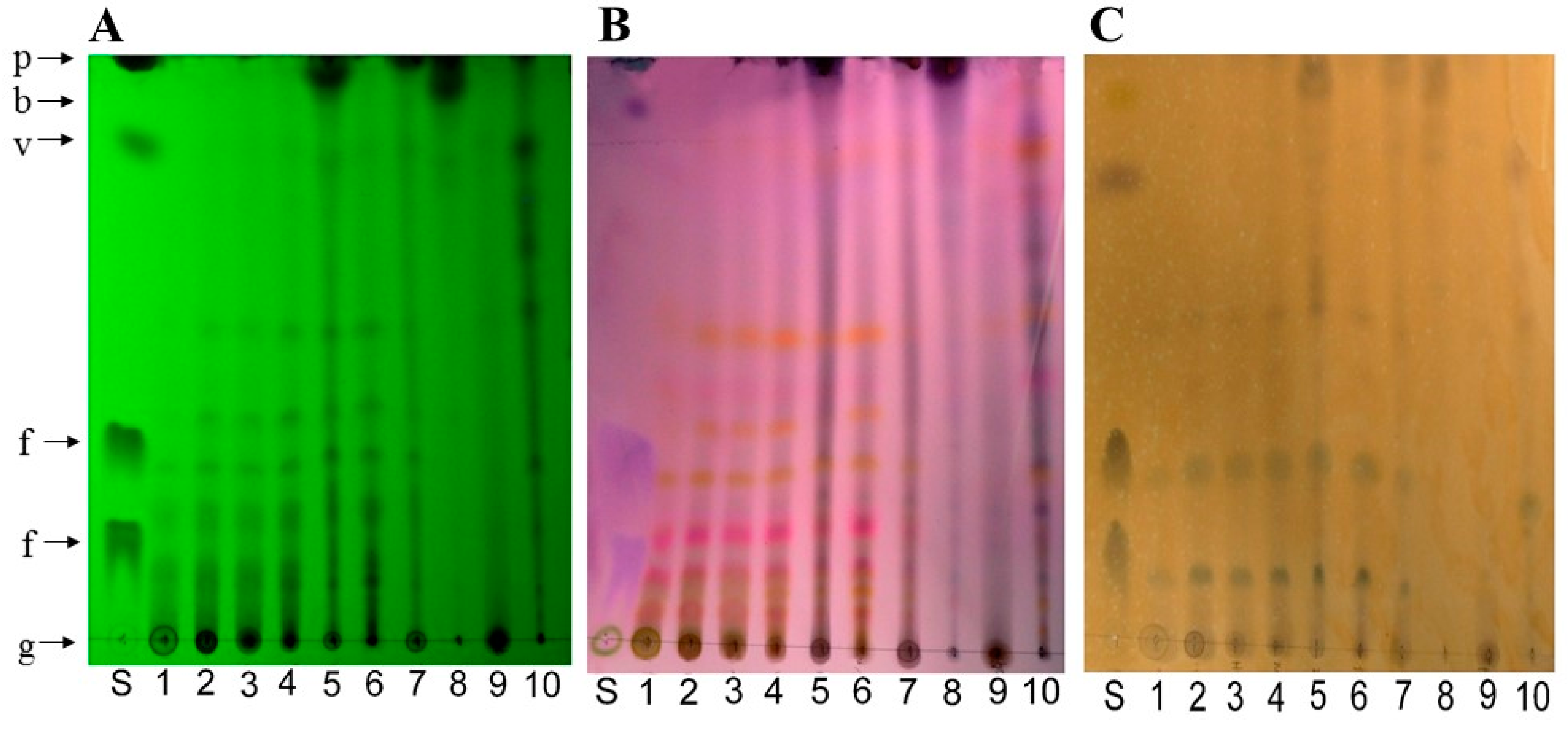
2.3. Thin Layer Chromatography (TLC) Analysis
2.4. Antimicrobial Assays
2.5. UPLC-QTOF-MS Characterization
3. Discussion
3.1. Composition and Extraction Yield Evaluation
3.2. Antimicrobial Activity
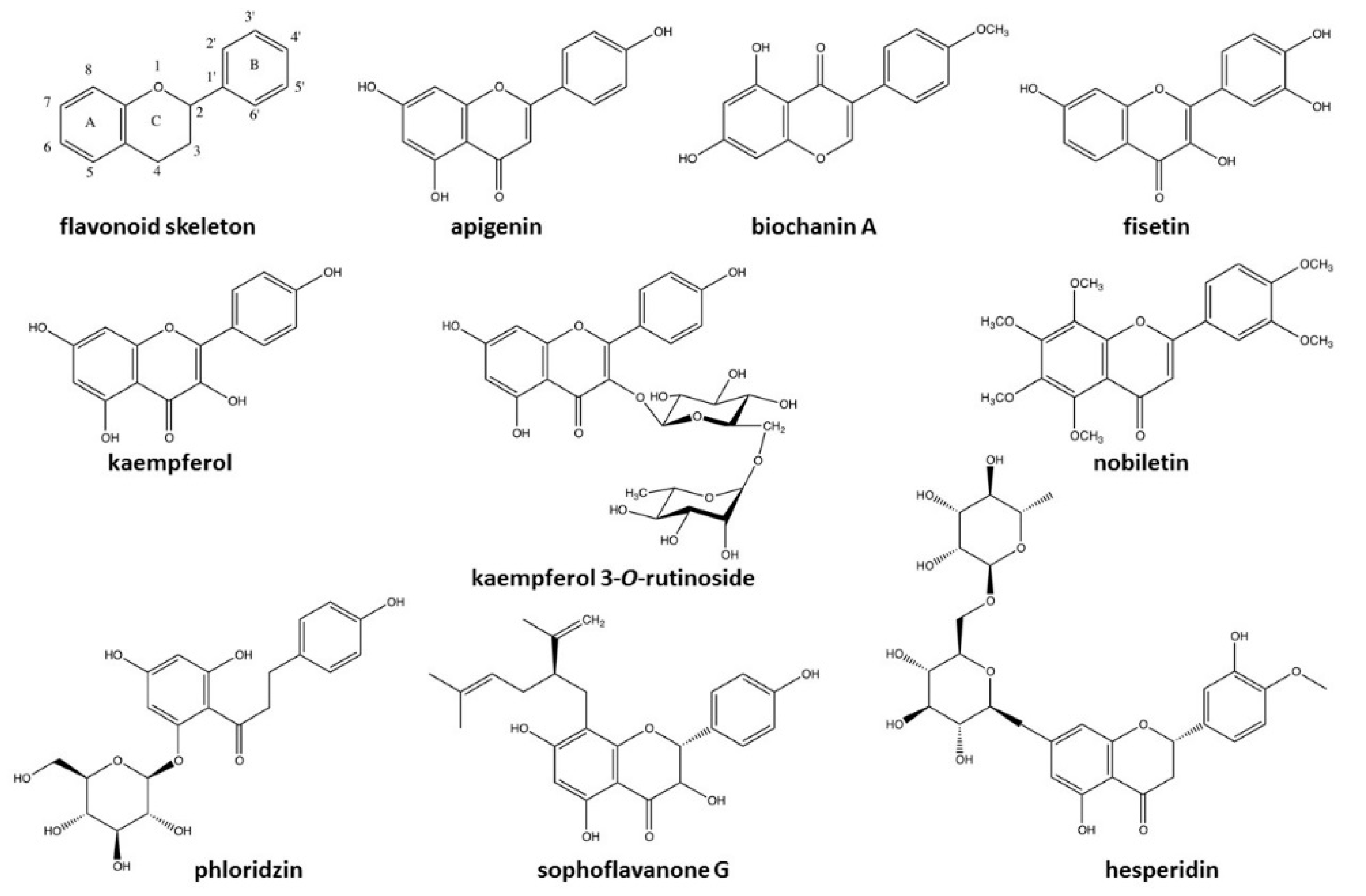
3.3. Chemical Characterization
4. Materials and Methods
4.1. Plant Material
4.2. Microbial Strains
4.3. Determination of Bark Composition and Extractive Yield per Fraction Size
4.4. Extractive Yield According to Molecular Polarity
4.5. Thin Layer Chromatography Method (TLC)
4.6. Antimicrobial Activity Test by Broth Microdilution Method
4.7. UPLC-QTOF-MS Method
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, M.L. Epidemiology of drug resistance: Implications for a post-antimicrobial era. Science (Wash.) 1992, 257, 1050–1055. [Google Scholar] [CrossRef]
- Gold, H.S.; Moellering, R.C., Jr. Antimicrobial-drug resistance. N. Engl. J. Med. 1996, 335, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Royer, M.; Houde, R.; Viano, Y.; Stevanovic, T. Non-wood Forest Products Based on Extractives-A New Opportunity for the Canadian Forest Industry Part 1: Hardwood Forest Species. J. Food Res. 2012, 1, 8. [Google Scholar] [CrossRef]
- Bouchard, M. Identification D’utilisations pour le Bouleau à Papier et Le peuplier Faux-Tremble; Enseignement Supérieur, Recherche, Science et Technologie: Quebec, QC, Canada, 2013; pp. 1–45. [Google Scholar]
- Diouf, P.N.; Stevanovic, T.; Cloutier, A. Antioxidant properties and polyphenol contents of trembling aspen bark extracts. Wood Sci. Technol. 2009, 43, 457–470. [Google Scholar] [CrossRef]
- Fernandez, M.P.; Watson, P.A.; Breuil, C. Gas chromatography–mass spectrometry method for the simultaneous determination of wood extractive compounds in quaking aspen. J. Chromatogr. A 2001, 922, 225–233. [Google Scholar] [CrossRef]
- Pal, D.; Singh, H.; Kumar, M. A preliminary study on the in vitro antioxidant activity of seeds of Aesculus indica and bark of Populus euphratica. Int. J. Pharm. Pharm. Sci. 2012, 4, 249–250. [Google Scholar]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-J.; Bao, J.-L.; Chen, X.-P.; Huang, M.; Wang, Y.-T. Alkaloids Isolated from Natural Herbs as the Anticancer Agents. Evid.-Based Complement. Altern. Med. 2012, 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic Compounds: Introduction; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar]
- Wink, M. Introduction: Biochemistry, physiology and ecological functions of secondary metabolites. Ann. Plant Rev. Biochem. Plant Second. Metabol. Second Ed. 2010, 40, 1–19. [Google Scholar]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 63–106. [Google Scholar]
- Celhay, C.; Mathieu, C.E.; Candy, L.; Vilarem, G.; Rigal, L. Aqueous extraction of polyphenols and antiradicals from wood by-products by a twin-screw extractor: Feasibility study. Comptes R. Chim. 2014, 17, 204–211. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, M.F. Biorefineries. In Algae Energy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 159–181. [Google Scholar]
- Janowiak, M.K.; Webster, C.R. Promoting ecological sustainability in woody biomass harvesting. J. For. 2010, 108, 16–23. [Google Scholar]
- SiliCycle Inc. SiliaPlate™-TLC Visualization Methods. Available online: http://www.silicycle.com/ca/products/thin-layer-chromatography-tlc-plates/siliaplate-tlc-visualization-methods (accessed on 13 July 2018).
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Abuelsaad, A.S.; Mohamed, I.; Allam, G.; Al-Solumani, A.A. Antimicrobial and immunomodulating activities of hesperidin and ellagic acid against diarrheic Aeromonas hydrophila in a murine model. Life Sci. 2013, 93, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Yu, Y.; Liang, Y.; Zeng, B. In vitro antioxidant and antimicrobial activities of the extract of Pericarpium Citri Reticulatae of a new Citrus cultivar and its main flavonoids. LWT-Food Sci. Technol. 2008, 41, 597–603. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. für Naturforschung C 2006, 61, 639–642. [Google Scholar] [CrossRef]
- Jeong, E.-Y.; Jeon, J.-H.; Lee, C.-H.; Lee, H.-S. Antimicrobial activity of catechol isolated from Diospyros kaki Thunb. roots and its derivatives toward intestinal bacteria. Food Chem. 2009, 115, 1006–1010. [Google Scholar] [CrossRef]
- Hwang, J.H.; Choi, H.; Hwang, I.-S.; Kim, A.R.; Woo, E.-R.; Lee, D.G. Synergistic Antibacterial and Antibiofilm Effect Between (+)-Medioresinol and Antibiotics In Vitro. Appl. Biochem. Biotechnol. 2013, 170, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Hwang, I.-S.; Liu, Q.-H.; Woo, E.-R.; Lee, D.G. (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie 2012, 94, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-D.; Jeong, M.-R.; Jeong, S.-I.; Lee, K.-Y. Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens. J. Microbiol. Biotechnol. 2007, 17, 858–864. [Google Scholar] [PubMed]
- Tsuchiya, H.; Iinuma, M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165. [Google Scholar] [CrossRef]
- Chi, Y.S.; Jong, H.G.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: Cyclooxygenases and lipoxygenases. Biochem. Pharmacol. 2001, 62, 1185–1191. [Google Scholar] [CrossRef]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J. Agric. Food Chem. 2007, 55, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Nguemeving, J.R.; Beng, V.P.; Azebaze, A.G.B.; Etoa, F.-X.; Meyer, M.; Bodo, B.; Nkengfack, A.E. Antimicrobial activity of the methanolic extracts and compounds from Vismia laurentii De Wild (Guttiferae). J. Ethnopharmacol. 2007, 109, 372–379. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wu, T.; Pan, S.; Xu, X. Antimicrobial mechanism of flavonoids against Escherichia coli ATCC 25922 by model membrane study. Appl. Surf. Sci. 2014, 305, 515–521. [Google Scholar] [CrossRef]
- Da Costa, M.P.; Bozinis, M.C.V.; Andrade, W.M.; Costa, C.R.; da Silva, A.L.; Alves de Oliveira, C.M.; Kato, L.; Fernandes, O.D.F.L.; Souza, L.K.H.; Silva, M.D.R.R. Antifungal and cytotoxicity activities of the fresh xylem sap of Hymenaea courbaril L. and its major constituent fisetin. BMC Complement. Altern. Med. 2014, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; McCutcheon, A.R.; Farmer, S.; Towers, G.H.N.; Hancock, R.E.W. Antimicrobial constituents of Rhus glabra. J. Ethnopharmacol. 1994, 42, 95–99. [Google Scholar] [CrossRef]
- Kukić, J.; Popović, V.; Petrović, S.; Mucaji, P.; Ćirić, A.; Stojković, D.; Soković, M. Antioxidant and antimicrobial activity of Cynara cardunculus extracts. Food Chem. 2008, 107, 861–868. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Giordano, S.; Ricciardi, L.; Ferrara, S.; Montesano, D.; Cobianchi, R.C.; Vuotto, M.; Ferrara, L. Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 2000, 71, S110–S116. [Google Scholar] [CrossRef]
- Basile, A.; Giordano, S.; López-Sáez, J.A.; Cobianchi, R.C. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Liang, J.-C.; Wang, X.-L.; Li, Z.-H.; Wang, W.; Guo, N.; Wu, X.-P.; Shen, F.-G.; Xing, M.-X.; Liu, L.-H. In vitro synergy of biochanin A and ciprofloxacin against clinical isolates of Staphylococcus aureus. Molecules 2011, 16, 6656–6666. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Herald, P.J.; Davidson, P.M. Antibacterial Activity of Selected Hydroxycinnamic Acids. J. Food Sci. 1983, 48, 1378–1379. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
- Merkl, R.; HRádkoVá, I.; FIlIp, V.; ŠMIdRkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.S.; Simões, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.R.; Alberto, M.; de Nadra, M.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heleno, S.A.; Ferreira, I.C.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Huq, A.; Jamal, J.A.; Stanslas, J. Ethnobotanical, phytochemical, pharmacological, and toxicological aspects of Persicaria hydropiper (L.) delarbre. Evid.-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Indwar, F.; Ignacimuthu, S. Antimicrobial activity of confertifolin from Polygonum hydropiper. Pharm. Biol. 2010, 48, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, antioxidant and antimicrobial activity of a new phloridzin derivative for dermo-cosmetic applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G. Antimicrobial activity of Calotropis procera Ait. (Asclepiadaceae) and isolation of four flavonoid glycosides as the active constituents. World J. Microbiol. Biotechnol. 2013, 29, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, G.; Sanogo, R.; Marino, A.; Aquino, R.; angelo, V.D.; Germano, M.P.; De Pasquale, R.; Pizza, C. Antimicrobial activity of Mitracarpus scaber extract and isolated constituents. Lett. Appl. Microbiol. 2000, 30, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Caponi, C.; Catalano, S.; Cioni, P.L.; Morelli, I. In vitro antimicrobial activity of extracts and isolated constituents of Rubus ulmifolius. J. Ethnopharmacol. 2002, 79, 165–168. [Google Scholar] [CrossRef]
- Čerňáková, M.; Košťálová, D. Antimicrobial activity of berberine—A constituent ofMahonia aquifolium. Folia Microbiol. 2002, 47, 375–378. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, X.; Pan, S.; Fang, Y.; Jiang, F.; Phillips, G.O.; Xu, X. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem. 2012, 132, 1883–1890. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O157: H7 in apple, pear and melon juices. Food Control 2009, 20, 105–112. [Google Scholar] [CrossRef]
- Gadang, V.; Hettiarachchy, N.; Johnson, M.; Owens, C. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a turkey frankfurter system. J. Food Sci. 2008, 73, M389–M394. [Google Scholar] [CrossRef] [PubMed]
- Eswaranandam, S.; Hettiarachchy, N.; Johnson, M. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157: H7, and Salmonella gaminara. J. Food Sci. 2004, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Kong, W.; Jin, C.; Zhao, Y.; Qu, Y.; Xiao, X. Microcalorimetric assay on the antimicrobial property of five hydroxyanthraquinone derivatives in rhubarb (Rheum palmatum L.) to Bifidobacterium adolescentis. Phytomedicine 2010, 17, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Yanwen, W.; Wenyuan, G.; Xiaohe, X.; Yi, L. Calorimetric investigation of the effect of hydroxyanthraquinones in Rheum officinale Baill on Staphylococcus aureus growth. Thermochim. Acta 2005, 429, 167–170. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-T.; Chen, P.-F.; Chang, S.-C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Smyth, T.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins. Int. J. Antimicrob. Agents 2009, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, T.G.; Darvell, L.I.; Jones, J.M.; Williams, P.T.; Fahmi, R.; Bridgwater, A.V.; Barraclough, T.; Shield, I.; Yates, N.; Thain, S.C.; et al. Influence of particle size on the analytical and chemical properties of two energy crops. Fuel 2007, 86, 60–72. [Google Scholar] [CrossRef]
- Liu, X.; Bi, X.T. Removal of inorganic constituents from pine bark and switchgrass. Fuel Process. Technol. 2011, 92, 1273–1279. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Fractioning and chemical characterization of bark of Betula pendula and Eucalyptus globulus. Ind. Crops Prod. 2013, 41, 299–305. [Google Scholar] [CrossRef]
- Koppejan, J.; Van Loo, S. The Handbook of Biomass Combustion and Co-Firing; Routledge: Abingdon, UK, 2012. [Google Scholar]
- Browne, T.; Paice, M.; Jemaa, N.; Paleologou, M.; Zhang, X.; Champoux, M. Le Bioraffinage Forestier: Possibilité Pour Les Entreprises Québécoises de Pâtes et Papiers; Ministère des Ressources Naturelles et de la Faune, Ed.; Gouvernement du Québec: Québec, QC, Canada, 2009; p. 40.
- Mehta, V.V.; Rajesh, G.; Rao, A.; Shenoy, R.; BH, M.P. Antimicrobial Efficacy of Punica granatum mesocarp, Nelumbo nucifera Leaf, Psidium guajava Leaf and Coffea Canephora Extract on Common Oral Pathogens: An In-vitro Study. J. Clin. Diagn. Res. 2014, 8, 65–68. [Google Scholar]
- Khan, N.; Abbasi, A.M.; Dastagir, G.; Nazir, A.; Shah, G.M.; Shah, M.M.; Shah, M.H. Ethnobotanical and antimicrobial study of some selected medicinal plants used in Khyber Pakhtunkhwa (KPK) as a potential source to cure infectious diseases. BMC Complement. Altern. Med. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anja Klančnik, S.P.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Vardar-Ünlü, G.; Silici, S.; Ünlü, M. Composition and in vitro antimicrobial activity of Populus buds and poplar-type propolis. World J. Microbiol. Biotechnol. 2008, 24, 1011–1017. [Google Scholar] [CrossRef]
- Dolab, J.G.; Lima, B.; Spaczynska, E.; Kos, J.; Cano, N.H.; Feresin, G.; Tapia, A.; Garibotto, F.; Petenatti, E.; Olivella, M.; et al. The Antimicrobial Activity of Annona emarginata (Schltdl.) H. Rainer and Most Active Isolated Compounds against Clinically Important Bacteria. Molecules 2018, 23, 1187. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar]
- Aguero, M.B.; Svetaz, L.; Sanchez, M.; Luna, L.; Lima, B.; Lopez, M.L.; Zacchino, S.; Palermo, J.; Wunderlin, D.; Feresin, G.E.; et al. Argentinean Andean propolis associated with the medicinal plant Larrea nitida Cav. (Zygophyllaceae). HPLC-MS and GC-MS characterization and antifungal activity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D. Amphotericin B: Spectrum and resistance. J. Antimicrob. Chemother. 2002, 49 (Suppl. 1), 7–10. [Google Scholar] [CrossRef] [PubMed]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 26, 1533–1538. [Google Scholar] [CrossRef]
- Slobodnikova, L.; Kost’alova, D.; Labudova, D.; Kotulova, D.; Kettmann, V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother. Res. PTR 2004, 18, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Assob, J.C.; Kamga, H.L.; Nsagha, D.S.; Njunda, A.L.; Nde, P.F.; Asongalem, E.A.; Njouendou, A.J.; Sandjon, B.; Penlap, V.B. Antimicrobial and toxicological activities of five medicinal plant species from Cameroon traditional medicine. BMC Complement. Altern. Med. 2011, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Buss, A.D. Natural products—The future scaffolds for novel antibiotics? Biochem. Pharmacol. 2006, 71, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; Brown, E.D. Strategies for target identification of antimicrobial natural products. Nat. Prod. Rep. 2016, 33, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Boziaris, I.; Nychas, G.-J.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Méndez, C.; Salas, J.A. Altering the glycosylation pattern of bioactive compounds. Trends Biotechnol. 2001, 19, 449–456. [Google Scholar] [CrossRef]
- Fu, X.; Albermann, C.; Jiang, J.; Liao, J.; Zhang, C.; Thorson, J.S. Antibiotic optimization via in vitro glycorandomization. Nat. Biotechnol. 2003, 21, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Gachon, C.M.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.P.; Clements, M.O.; Foster, S.J. Characterization of the Starvation-Survival Response of Staphylococcus aureus. J. Bacteriol. 1998, 180, 1750–1758. [Google Scholar] [PubMed]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L. Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Boik, J.; Kirakosyan, A.; Kaufman, P.B.; Seymour, E.M.; Spelman, K. Interactions of Bioactive Plant Metabolites: Synergism, Antagonism, and Additivity. In Recent Advances in Plant Biotechnology; Springer: Boston, MA, USA, 2009; pp. 213–230. [Google Scholar]
- Lindroth, R.; Osier, T.; Barnhill, H.; Wood, S. Effects of genotype and nutrient availability on phytochemistry of trembling aspen (Populus tremuloides Michx.) during leaf senescence. Biochem. Syst. Ecol. 2002, 30, 297–307. [Google Scholar] [CrossRef]
- Osier, T.L.; Hwang, S.-Y.; Lindroth, R.L. Within- and between-year variation in early season phytochemistry of quaking aspen (Populus tremuloides Michx.) clones. Biochem. Syst. Ecol. 2000, 28, 197–208. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory Biomass Compositional Analysis Laboratory Procedures. Available online: https://www.nrel.gov/bioenergy/biomass-compositional-analysis.html (accessed on 13 July 2018).
- Yubin, J.; Miao, Y.; Bing, W.; Yao, Z. The extraction, separation and purification of alkaloids in the natural medicine. J. Chem. Pharm. Res. 2014, 6, 338–345. [Google Scholar]
- Prado, J.M.; Meireles, M.A.A. Supercritical fluid extraction of lemon verbena (Aloysia triphylla): Kinetics, scale-up and chemical composition. In Proceedings of the 12th European Meeting on Supercritical Fluids, Graz, Austria, 9–12 May 2010. [Google Scholar]
- Jia, Z.; Tian, C. Quantitative determination of polyethylene glycol with modified Dragendorff reagent method. Desalination 2009, 247, 423–429. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing filamentous fungi, approved standard. In CLSI Document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. In CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Extract | E. coli | S. enterica | P. aeruginosa | S. aureus | E. faecalis | A. niger | C. albicans | S. cerevisiae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC a | MBC b | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | MIC | MFC | MIC | MFC | |
| Water | 1.67 | − c | 0.83 | − | 0.83 | 4.44 | 1.67 | 1.67 | 1.67 | − | 0.83 | − | 1.67 | − | 2.22 | 4.44 |
| Methanol | − | − | − | − | − | − | 4.44 | − | − | − | 1.67 | − | − | − | 2.22 | 4.44 |
| Ethanol | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Acetone | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Methylene chloride | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Ethyl acetate | − | − | − | − | 4.44 | − | − | − | − | − | − | − | − | − | − | − |
| chloroform | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Hexane | − | − | 1.67 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Water-ethanol | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Acid-base | 4.44 | − | − | − | 2.22 | − | − | − | − | − | 2.22 | − | 4.44 | − | − | − |
| QAC d | 2.60 e | 5.21 e | 0.651 e | 0.651 e | 10.4 e | 10.4 e | 5.21 e | 5.21 e | 2.60 e | 2.60 e | 10.4 e | 10.4 e | 5.21 e | 5.21 e | 2.60 e | 2.60 e |
| Compound Class | Proposed Compound a | % Area b | RT c (min) | Exact Mass (m/z) | |
|---|---|---|---|---|---|
| [M + H]+ d | [M − H]− d | ||||
| Polyphenols | Uvarinol | 4.44 | 13.01 | 573.1666 | |
| Acacetin | 4.37 | 15.73 | 285.0872 | ||
| Cyanidin | 2.22 | 7.71 | 288.017 | ||
| Chamuvaritin | 1.8 | 9.70 | 451.1318 | ||
| Hesperidin | 1.76 | 13.32 | 301.084 | ||
| Dimethylquercetin | 1.57 | 6.06 | 329.1043 | ||
| Catechol | 1.22 | 5.99 | 109.0582 | ||
| Acetylglycitin | 0.95 | 9.09 | 487.1998 | ||
| Phenolic acids | Diferuloyquinic acid | 4.19 | 12.41 | 543.1303 | |
| Coumaric acid | 1.73 | 7.9 | 163.0654 | ||
| Hydroxybenzoic acid | 1.13 | 9.96 | 137.0445 | ||
| Coumaroylquinic acid | 1.05 | 5.17 | 337.1691 | ||
| Caffeic acid | 0.78 | 1.31 | 179.0711 | ||
| Terpenoids | Arbusculin A | 1.38 | 8.15 | 251.16 | |
| Confertifolin | 1.36 | 6.57 | 235.1504 | ||
| Palustradiene | 0.76 | 8.93 | 273.2144 | ||
| Sugars | Galloyl glucose | 4.82 | 5.29 | 331.1157 | |
| D-Ribofuranose | 1.53 | 6.11 | 150.869 | ||
| Glycosylated compounds | Phloridzin | 4.76 | 10.93 | 435.1344 | |
| Apigenin-glucoside | 4.22 | 10.42 | 431.14 | ||
| Grandidentatin | 3.89 | 9.94 | 423.1767 | ||
| Coumaric acid glucoside | 3.45 | 6.21 | 325.0998 | ||
| Naringenin-glucoside | 1.36 | 9.34 | 433.1198 | ||
| Luteolin-hexoside | 0.81 | 11.49 | 447.1463 | ||
| Kaempferol-hexoside | 0.75 | 10.72 | 461.1678 | ||
| Kaempferol-hexoside | 0.71 | 9.67 | 447.1667 | ||
| Alkaloids | Dihydrozeatin | 3.75 | 10.44 | 222.1829 | |
| isoquinoline-1.5-diol | 3.14 | 5.34 | 161.7838 | ||
| (−)-Hygroline | 1.54 | 7.15 | 144.1 | ||
| Others | Butonate | 5.47 | 13.95 | 326.9853 | |
| 4-Hydroxybenzaldehyde | 5.44 | 9.83 | 122.8692 | ||
| 1,2,4-Trimethylbenzene | 4.19 | 15.79 | 121.5811 | ||
| Hydroxyanthraquinone | 4.05 | 10.95 | 225.053 | ||
| Gluconic acid | 2.06 | 13.98 | 195.1299 | ||
| Adipic acid | 1.65 | 5.22 | 146.8463 | ||
| Nobiletin | 0.98 | 1.45 | 401.1542 | ||
| Malic acid | 0.97 | 8.67 | 133.4128 | ||
| Thiodiacetic acid | 0.89 | 13.69 | 150.9699 | ||
| 1.4-Naphthoquinone | 0.83 | 1.52 | 159.0468 | ||
| Compound Class | Proposed Compound a | % Area b | RT c (min) | Exact Mass (m/z) | |
|---|---|---|---|---|---|
| [M + H]+ d | [M − H]− d | ||||
| Polyphenols | 4-prenylresveratrol | 7.26 | 14.4 | 295.1533 | |
| Kaempferol | 5.03 | 15.6 | 287.0954 | ||
| Isorhamnetin | 4.39 | 10.63 | 315.1832 | ||
| 3-methoxyapigenin | 3.49 | 15.9 | 301.0837 | ||
| Cirsimaritin | 3.13 | 11.46 | 313.1609 | ||
| Pinobanksin | 3.11 | 12.2 | 273.0878 | ||
| Epirosmanol | 2.58 | 5.08 | 345.1393 | ||
| Medioresinol | 2.19 | 7.34 | 387.1865 | ||
| Kaempferide | 2.12 | 14.1 | 299.187 | ||
| Kaempferol | 1.98 | 15.56 | 285.0911 | ||
| Catechol | 1.63 | 5.65 | 109.0384 | ||
| Sophoraflavanone G | 1.45 | 10.13 | 423.1942 | ||
| 5-tricosenylresorcinol | 1.39 | 10.25 | 431.1579 | ||
| Kaempferide | 1.21 | 15.93 | 299.0782 | ||
| Fisetin | 1.08 | 12.4 | 287.0953 | ||
| Apigenin | 0.92 | 13.6 | 271.1084 | ||
| Biochanin A | 0.92 | 15.7 | 285.0903 | ||
| Glepidotin | 0.68 | 15.23 | 297.175 | ||
| Apigenin | 0.51 | 12.2 | 271.0766 | ||
| Phenolic acids | 4-hydroxybenzoic acid | 2.09 | 9.15 | 137.0339 | |
| 3-hydroxybenzoic acid | 1.77 | 5.58 | 137.0339 | ||
| Caffeic acid | 0.94 | 1.18 | 179.0467 | ||
| 4-hydroxyphenylacetic acid | 0.83 | 4.35 | 151.0183 | ||
| Hydroxycaffeic acid | 0.67 | 1.21 | 195.0708 | ||
| Vanilic acid | 0.59 | 6.37 | 167.0509 | ||
| Terpenoids | Trilobolide | 1.13 | 11.51 | 521.2712 | |
| Phytuberin | 0.87 | 15.31 | 293.1385 | ||
| Sugars | D-Rhamnose | 2.64 | 1 | 165.0511 | |
| Chitobiose | 1.01 | 9.8 | 425.1993 | ||
| D-Glucose | 0.65 | 1 | 181.0349 | ||
| Glycosylated compounds | Kaempferol 3-O-rutinoside | 3.15 | 11.08 | 593.2896 | |
| Salicin | 2.37 | 11.4 | 287.1035 | ||
| Syringin | 1.93 | 11.9 | 373.1422 | ||
| Myricetin 3-O-arabinoside | 1.74 | 5.58 | 449.1708 | ||
| Arbutin | 1.65 | 12.2 | 271.0804 | ||
| Jaceidin 4′-O-glucuronide | 1.47 | 9.67 | 535.2496 | ||
| Ferulic acid 4-O-glucoside | 1.33 | 9.93 | 355.1625 | ||
| Fisetin 8-C-glucoside | 1.19 | 9.1 | 449.1325 | ||
| Quercetin-3-O-glucuronide | 0.8 | 9.3 | 477.251 | ||
| Gallic acid 4-O-glucoside | 0.8 | 14.4 | 331.1295 | ||
| Quercetin-3-O-glucuronide | 0.68 | 10.16 | 477.251 | ||
| Alkaloids | Berberine | 0.5 | 11.9 | 337.1196 | |
| Others | Oleamide | 9.61 | 26.6 | 282.2869 | |
| Octadecyl | 3.38 | 23.8 | 254.2584 | ||
| 1-Heptadecanamine | 2.89 | 26 | 256.276 | ||
| 4-Hydroxybenzaldehyde | 1.47 | 9.6 | 123.0455 | ||
| 4-Hydroxybenzaldehyde | 1.11 | 6.85 | 121.0385 | ||
| Coumarin | 1.03 | 7.6 | 147.0472 | ||
| 4-nitrophenol | 0.96 | 34.9 | 139.9936 | ||
| Benzoin | 0.89 | 5.1 | 213.1013 | ||
| Simazine | 0.76 | 1 | 202.0671 | ||
| Diosgenin | 0.75 | 7.2 | 415.276 | ||
| 1.4-Naphthoquinone | 0.55 | 3.14 | 157.0664 | ||
| Compound Class | Compound Name a | Presence b | Ref. | |
|---|---|---|---|---|
| Water Extract | Methanol Extract | |||
| Polyphenols | Hesperidin | − | + | [25,26] |
| Catechol | + | + | [27,28] | |
| Medioresinol | ++ | − | [29,30] | |
| Sophoraflavanone G | + | − | [31,32,33] | |
| Kaempherol | +++ | − | [34,35,36] | |
| Fisetin | + | − | [37,38] | |
| Apigenin | + | − | [39,40,41] | |
| Biochanin A | + | − | [36,42] | |
| Phenolic acids | Coumaric acid | − | + | [43,44,45] |
| Caffeic acid | + | + | [46,47,48,49,50] | |
| Vanillic acid | + | − | [46,50,51,52] | |
| 4-hydroxybenzoic acid | ++ | − | [46,53] | |
| 3-hydroxybenzoic acid | + | − | [51,52] | |
| Terpenoids | Confertifolin | [54,55] | ||
| Glycosylated compound | Phloridzin | − | +++ | [49,56,57] |
| Kaempferol 3-O-rutinoside | ++ | - | [58,59,60] | |
| Alkaloids | Berberine | + | − | [61,62,63] |
| Nobiletin | − | + | [26,64] | |
| Others | Malic acid | − | + | [65,66,67] |
| Hydroxyanthraquinone | − | +++ | [67,68,69] | |
| 4-hydroxybenzaldehyde | + | +++ | [70,71] | |
| Coumarin | + | - | [71,72] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
St-Pierre, A.; Blondeau, D.; Lajeunesse, A.; Bley, J.; Bourdeau, N.; Desgagné-Penix, I. Phytochemical Screening of Quaking Aspen (Populus tremuloides) Extracts by UPLC-QTOF-MS and Evaluation of their Antimicrobial Activity. Molecules 2018, 23, 1739. https://doi.org/10.3390/molecules23071739
St-Pierre A, Blondeau D, Lajeunesse A, Bley J, Bourdeau N, Desgagné-Penix I. Phytochemical Screening of Quaking Aspen (Populus tremuloides) Extracts by UPLC-QTOF-MS and Evaluation of their Antimicrobial Activity. Molecules. 2018; 23(7):1739. https://doi.org/10.3390/molecules23071739
Chicago/Turabian StyleSt-Pierre, Annabelle, Dorian Blondeau, André Lajeunesse, Julien Bley, Nathalie Bourdeau, and Isabel Desgagné-Penix. 2018. "Phytochemical Screening of Quaking Aspen (Populus tremuloides) Extracts by UPLC-QTOF-MS and Evaluation of their Antimicrobial Activity" Molecules 23, no. 7: 1739. https://doi.org/10.3390/molecules23071739






