DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses
Abstract
:1. Introduction
2. Results and Discussion
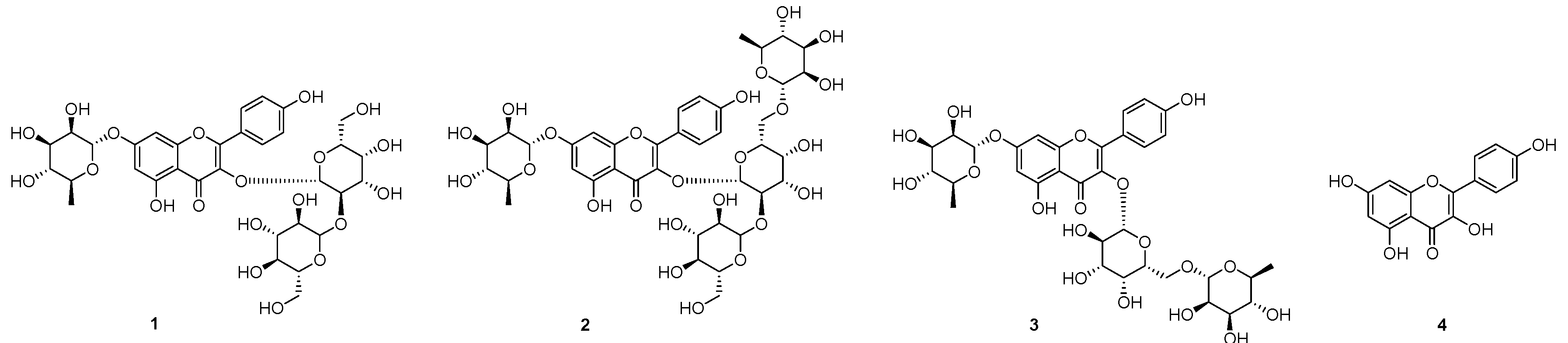
2.1. Isolation of Compounds 1–3 from the Seeds of L. culinaris
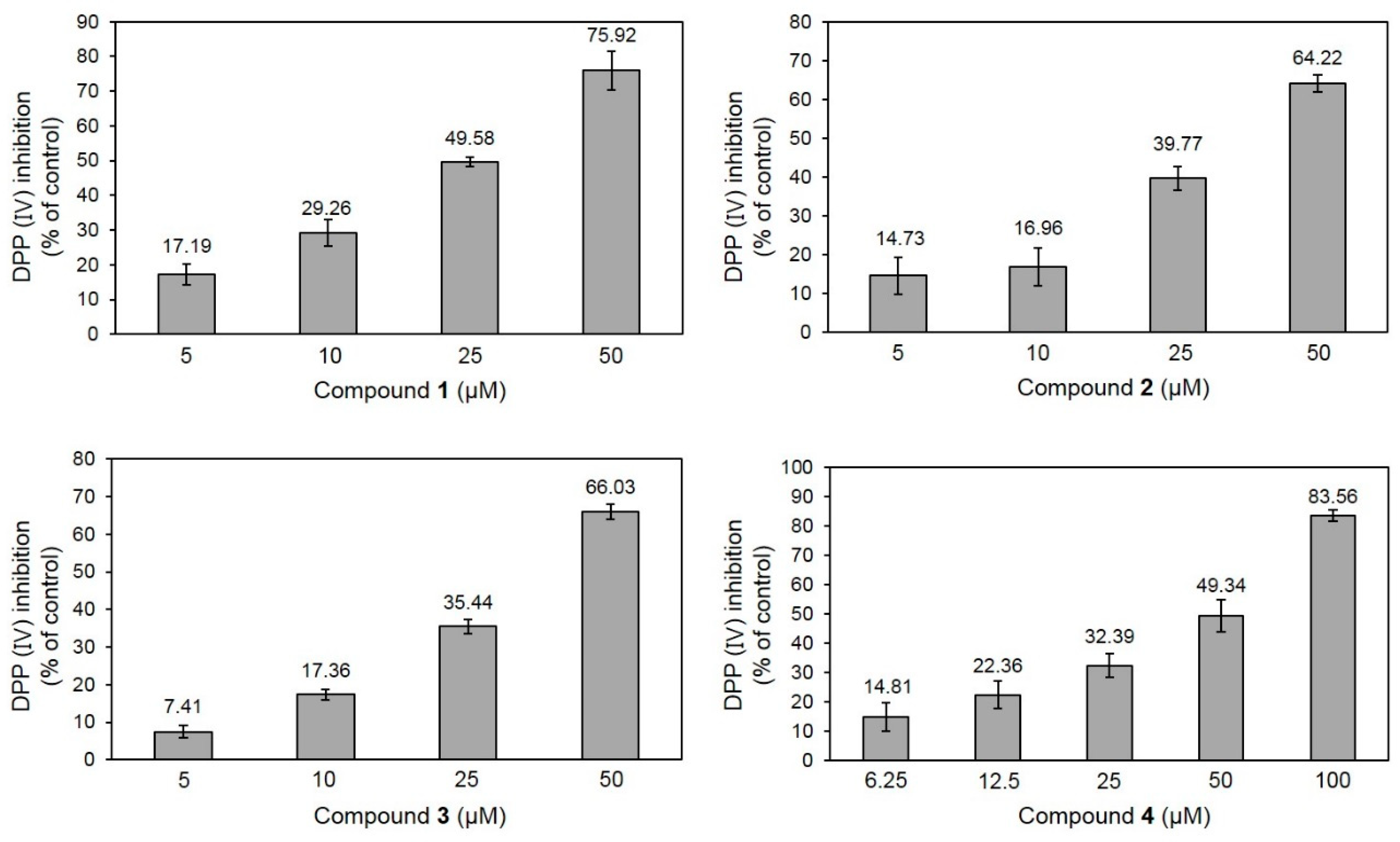
2.2. DPP-IV Inhibitory Activity of 1–4
2.3. Molecular Docking Analysis of Compounds 1–4
3. Materials and Methods
3.1. General Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. DPP-IV–Inhibitory Activity Assay
3.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, S.I. Deconstructing type 2 diabetes. Cell 1999, 97, 9–12. [Google Scholar] [CrossRef]
- Nauck, M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 2016, 18, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Diabetes Mellitus Fact Sheet. Available online: http://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 23 July 2018).
- Rasmussen, H.B.; Branner, S.; Wiberg, F.C.; Wagtmann, N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat. Struct. Biol. 2003, 10, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul. Pept. 1999, 85, 9–24. [Google Scholar] [CrossRef]
- Langley, A.K.; Suffoletta, T.J.; Jennings, H.R. Dipeptidyl peptidase IV inhibitors and the incretin system in type 2 Diabets Mellitus. Pharmacotheraphy 2007, 27, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Vilsboll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. No reactive hypoglycaemia in Type 2 diabetic patients after subcutaneous administration of GLP-1 and intravenous glucose. Diabetic Med. 2001, 18, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Fatty acid, carotenoid and tocopherol compositions of 20 Canadian lentil cultivars and synergistic contribution to antioxidant activities. Food Chem. 2014, 161, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on a-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Yu, H.; Xia, L.; Ng, T.B. Lectin from green speckled lentil seeds (Lens culinaris) triggered apoptosis in nasopharyngeal carcinoma cell lines. Chin. Med. 2015, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.; Ali, A.; Ali, M. Isolation of antioxidant phytoconstituents from the seeds of Lens culinaris Medik. Food Chem. 2015, 175, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Tsopmo, A.; Muir, A.D. Chemical profiling of lentil (Lens culinaris Medik.) cultivars and isolation of compounds. J. Agric. Food Chem. 2010, 58, 8715–8721. [Google Scholar] [CrossRef] [PubMed]
- Sagratini, G.; Zuo, Y.; Caprioli, G.; Cristalli, G.; Giardinà, D.; Maggi, F.; Molin, L.; Ricciutelli, M.; Traldi, P.; Vittori, S. Quantification of soyasaponins I and βg in Italian lentil seeds by solid-phase extraction (SPE) and high-performance liquid chromatography-mass spectrometry (HPLC-MS). J. Agric. Food Chem. 2009, 57, 11226–11233. [Google Scholar] [CrossRef] [PubMed]
- Kite, G.C.; Veitch, N.C.; Boalch, M.E.; Lewis, G.P.; Leon, C.J.; Simmonds, M.S. Flavonol tetraglycosides from fruits of Styphnolobium. japonicum (Leguminosae) and the authentication of Fructus Sophorae and Flos Sophorae. Phytochemistry 2009, 70, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, M.; Mabry, T.J.; Dixon, R.A. Flavonol glycosides from Cephalocereus. senilis. Phytochemistry 1994, 36, 229–231. [Google Scholar] [PubMed]
- Srivastava, S.; Shree, P.; Tripathi, Y.B. Active phytochemicals of Pueraria tuberosa for DPP-IV inhibition: In silico and experimental approach. J. Diabetes Metab. Disord. 2017, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; Mejia, E.G. Berry and citrus phenolic compounds inhibit dipeptidyl peptidase IV: Implications in diabetes management. Evid. Based Complement. Alternat. Med. 2013, 479505. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Zhu, J.; Li, B.; Li, Z.; Zhu, W.; Shi, J.; Jia, Q.; Li, Y. Recent progress in natural products as DPP-4 inhibitors. Future Med. Chem. 2015, 7, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Kalhotra, P.; Chittepu, V.C.S.R.; Osorio-Revilla, G.; Gallardo-Velázquez, T. Structure-activity relationship and molecular docking od natural product library reveal chrysin as a novel dipeptidyl peptidase-4 (DPP-4) inhibitors: An integrated in silico and in vitro study. Molecules 2018, 23, 1368. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L. Identification of Isoquercitrin as an Inhibitor of DPP-IV: Implication for Insulin Secretion and Hyperglycemic in Type 2 Diabetes Mice. Ph.D. Thesis, Jilin University, Changchun, China, 2013. [Google Scholar]
- Parmar, H.S.; Jain, P.; Chauhan, D.S.; Bhinchar, M.K.; Munjal, V.; Yusuf, M.; Choube, K.; Tawani, A.; Tiwani, V.; Manivannan, E.; et al. DPP-IV inhibitory potential of narningin: An in silico, in vitro, in vivo study. Diabetes Res. Clin. Pract. 2012, 97, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wang, L.; Beconi, M.; Eiermann, G.J.; Fisher, M.H.; He, H.; Hickey, G.J.; Kowalchick, J.E.; Leiting, B.; Lyons, K.; et al. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: A potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2005, 48, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, O.; Tanwar, L.; Shaquiquzzaman, M.; Alam, M.M.; Akhter, M. Structure based virtual screening of MDPI database: discovery of structurally diverse and novel DPP-IV inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 3447–3451. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2017–1; Glide, Schrödinger, LLC: New York, NY, USA, 2017.
Sample Availability: Samples of the compounds are not available from the authors. |
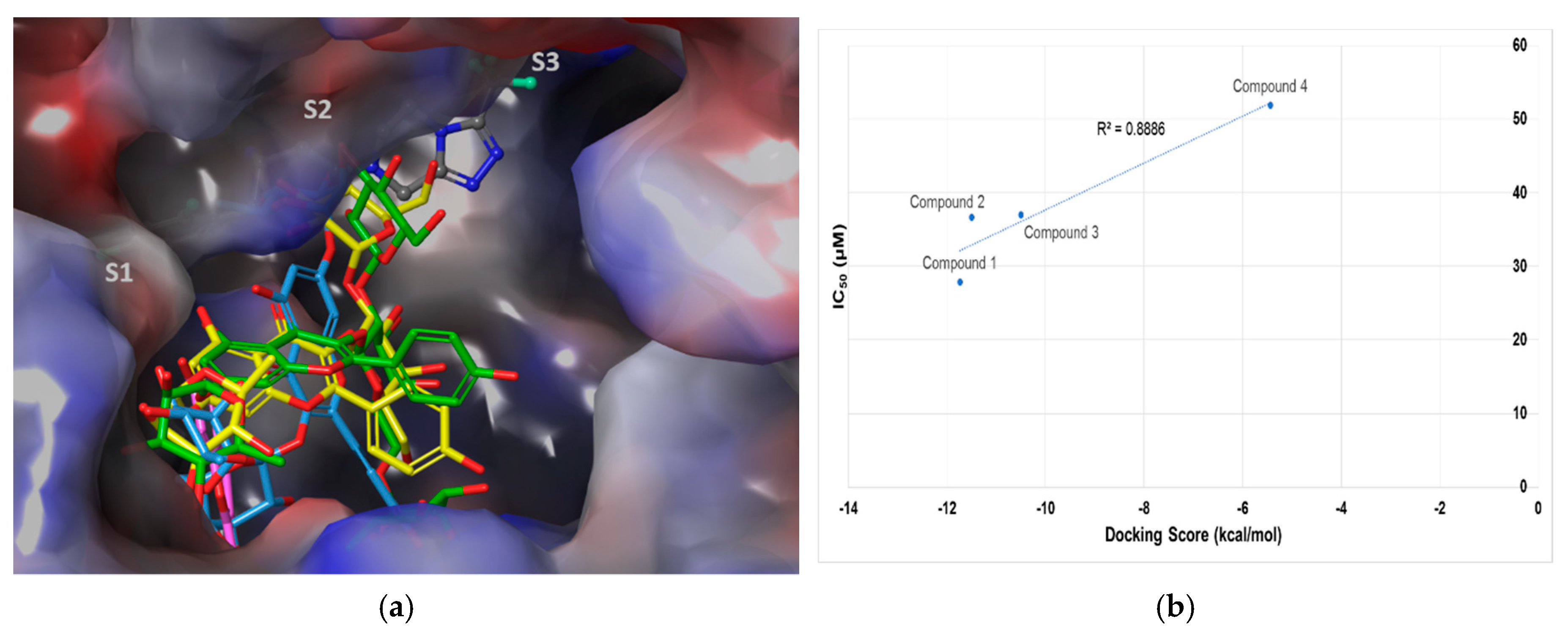
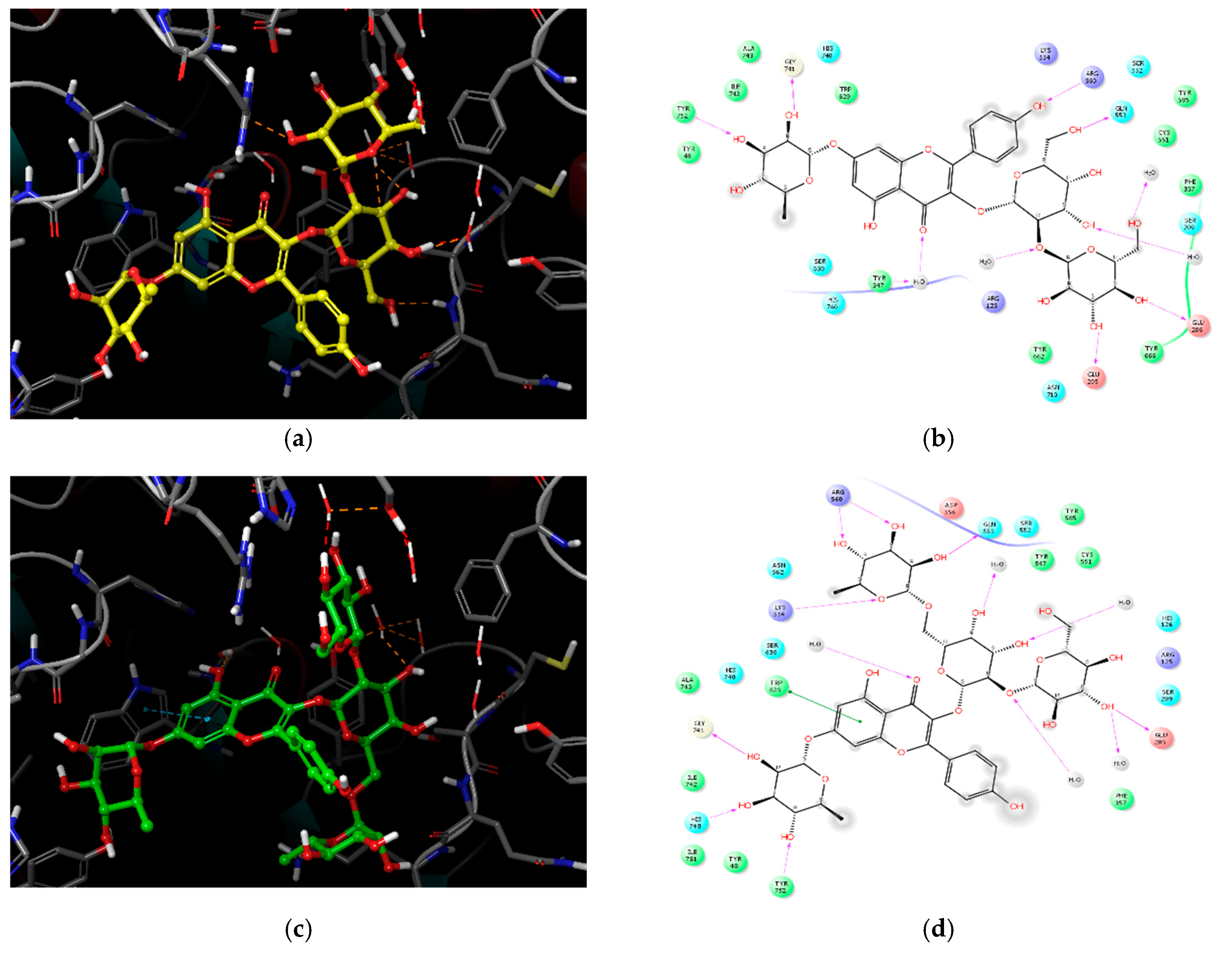
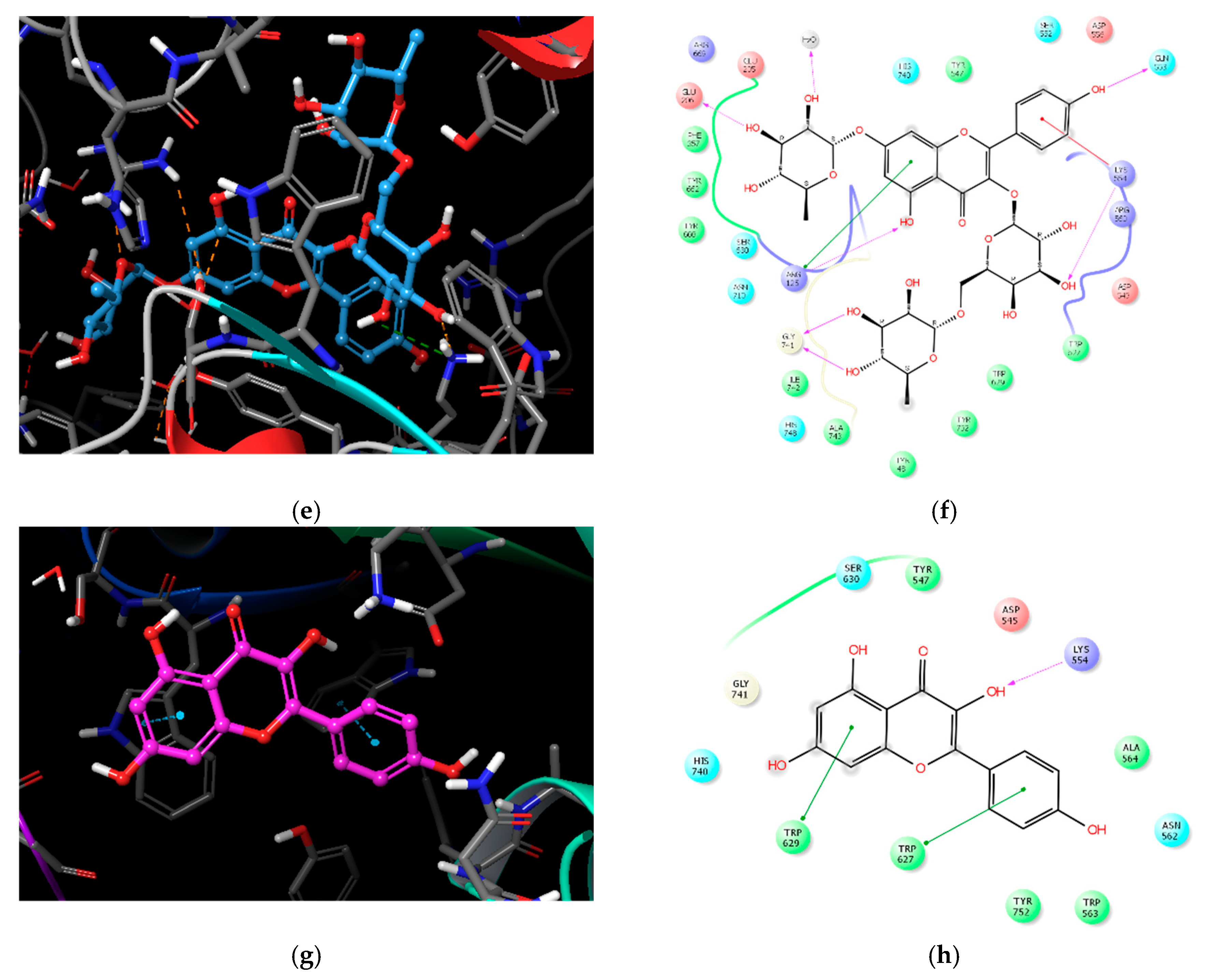
| Compound | IC50 (μM) 1 | Number of OH Groups | Docking Score (kcal/mol) | Interacting Residues (≤4.0 Å) 2 |
|---|---|---|---|---|
| 1 | 27.89 ± 1.29 | 12 | −11.737 | H-bond: E205, E206, Q533, Y547 (water-mediated), R560, G741, Y752, HOH1605, HOH1617, HOH1927, HOH1957 VDW: W629, S630 |
| 2 | 36.52 ± 0.78 | 14 | −11.499 | H-bond: E205, Q553, K554, R560 (2), G741, H748, Y752, HOH1582, HOH1617, HOH1732, HOH1927, HOH1957 π–π: W629 |
| 3 | 37.01 ± 1.40 | 11 | −10.494 | H-bond: R125, E206, Q553, K554, G741(2), HOH1582 π–cation: K554 VDW: R125 |
| 4 | 51.69 ± 4.83 | 4 | −5.439 | H-bond: K554 π–π: W627, W629 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-R.; Kim, H.Y.; Choi, I.; Kim, J.-B.; Jin, C.H.; Han, A.-R. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules 2018, 23, 1998. https://doi.org/10.3390/molecules23081998
Kim B-R, Kim HY, Choi I, Kim J-B, Jin CH, Han A-R. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules. 2018; 23(8):1998. https://doi.org/10.3390/molecules23081998
Chicago/Turabian StyleKim, Bo-Ram, Hyo Young Kim, Inhee Choi, Jin-Baek Kim, Chang Hyun Jin, and Ah-Reum Han. 2018. "DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses" Molecules 23, no. 8: 1998. https://doi.org/10.3390/molecules23081998





