Synthesis, Spectroscopic Analysis and Assessment of the Biological Activity of New Hydrazine and Hydrazide Derivatives of 3-Formylchromone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
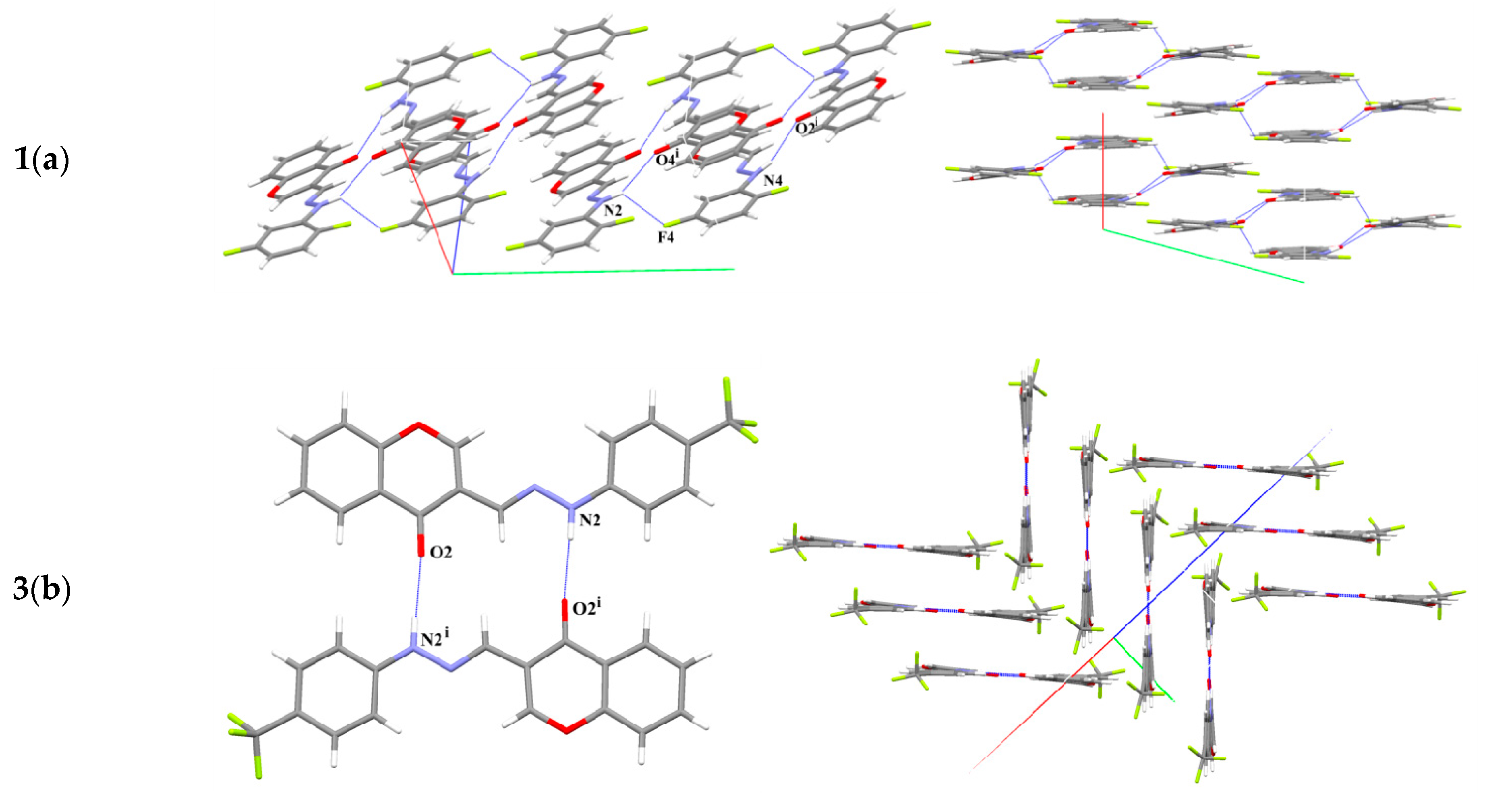
2.2. X-ray Diffraction Studies
2.3. Initial Determining the Sensitivity of Microorganisms to the Obtained Chemical Compounds
2.4. Cell Proliferation Study
2.5. Statistical Analysis of Cell Proliferation Results
3. Experimental Section
3.1. General Information
3.2. Synthesis of Hydrazine and Hydrazide Derivatives of 3-Formylchromone
3.3. Synthesis of Cu(II) 3-Formylchromone Hydrazide Complex
3.4. Spectral and Elemental Analysis
3.5. X-ray Diffraction Studies
3.6. Research on Antimicrobial Potential (Initial Determination)
3.7. XTT-Assay—Cell Culture
3.8. XTT Cytotoxicity Assay
3.9. Statistical Analysis of Cell Proliferation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Haddar, A.; Sellimi, S.; Ghannouchi, R.; Alvarez, O.M.; Nasri, M.; Bougatef, A. Functional, antioxidant and film-forming properties of tuna-skin gelatin with a brown algae extract. Int. J. Biol. Macromol. 2012, 51, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Brauers, G.; Ebel, R.; Wray, V.; Berg, A.; Proksch, P. Novel chromone derivatives from the fungus Aspergillus versicolor isolated from the marine sponge Xestospongia exigua. J. Nat. Prod. 2003, 66, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, S.; Niu, S.; Guo, L.; Chen, X.; Che, Y. Isoprenylated chromone derivatives from the plant endophytic fungus Pestalotiopsis fici. J. Nat. Prod. 2009, 72, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.V.; Mulchandani, N.B. Structure reinvestigation of conyzorigun, a new chromone from Ageratum conyzoides. J. Chem. Soc. Perkin Trans. 1984, 0, 2945–2947. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Curir, P.; Galeotti, F.; Dolci, M.; Barile, E.; Lanzotti, V. Pavietin, a coumarin from Aesculus pavia with antifungal activity. J. Nat. Prod. 2007, 70, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Das, R.; Sarkhel, S.; Mishra, R.; Mukherjee, S.; Bhattacharya, S.; Gomes, A. Herbs and herbal constituents active against snake bite. Indian J. Exp. Biol. 2010, 48, 865–878. [Google Scholar] [PubMed]
- Ibrahim, M.A.; El-Mahdy, K.M. Synthesis and Antimicrobial activity of some new heterocyclic Schiff bases derived from 2-amino-3-formylchromone. Phosphorus Sulfur 2009, 184, 2945–2958. [Google Scholar] [CrossRef]
- Nawrot-Modranka, J.; Nawrot, E.; Graczyk, J. In vivo antitumor, in vitro antibacterial activity and alkylating properties of phosphorohydrazine derivatives of coumarin and chromone. Eur. J. Med. Chem. 2006, 41, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Ahmad, A.; Ambreen, N.; Amyn, A.; Perveen, S.; Khan, S.A.; Choudhary, M.I. Schiff bases of 3-formylchromones as antibacterial, antifungal, and phytotoxic agents (Supplementry Table). Lett. Drug Des. Discov. 2009, 6, 363–373. [Google Scholar] [CrossRef]
- Dias, M.M.; Machado, N.F.L.; Marques, M.P.M. Dietary chromones as antioxidant agents—the structural variable. Food Funct. 2011, 2, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Łazarenkow, A.; Nawrot-Modranka, J.; Brzezińska, E.; Krajewska, U.; Różalski, M. Synthesis, preliminary cytotoxicity evaluation of new 3-formylchromone hydrazones and phosphorohydrazone derivatives of coumarin and chromone. Med. Chem. Res. 2012, 21, 1861–1868. [Google Scholar] [CrossRef]
- Kawase, M.; Tanaka, T.; Kan, H.; Tani, S.; Nakashima, H.; Sakagami, H. Biological activity of 3-formylchromones and related compounds. In Vivo 2007, 21, 829–834. [Google Scholar] [PubMed]
- Khan, K.M.; Ambreen, N.; Mughal, U.R.; Jalil, S.; Perveen, S.; Choudhary, M.I. 3-Formylchromones: Potential antiinflammatory agents. Eur. J. Med. Chem. 2010, 45, 4058–4064. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, J.J.; Wang, J.; Yang, X.L.; Cai, P.; Liu, Q.H.; Kong, L.Y.; Wang, X.B. Synthesis and pharmacological evaluation of novel chromone derivatives as balanced multifunctional agents against Alzheimer’s disease. Bioorg. Med. Chem. 2017, 25, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.K. Chemistry of 4-oxo-4H-[1]benzopyran-3-carboxaldehyde. J. Heterocycl. Chem. 1983, 20, 1437–1445. [Google Scholar] [CrossRef]
- El-Shaaer, H.M.; Foltínová, P.; Lácová, M.; Chovancová, J.; Stankovičová, H. Synthesis, antimicrobial activity and bleaching effect of some reaction products of 4-oxo-4H-benzopyran-3-carboxaldehydes with aminobenzothiazoles and hydrazides. Farmaco 1998, 53, 224–232. [Google Scholar] [CrossRef]
- Elsayed, S.A.; Butler, I.S.; Claude, B.J.; Mostafa, S.I. Synthesis, characterization and anticancer activity of 3-formylchromone benzoylhydrazone metal complexes. Transit. Met. Chem. 2015, 40, 179–187. [Google Scholar] [CrossRef]
- Philip, J.E.; Antony, S.A.; Eeettinilkunnathil, S.J.; Kurup, M.R.P.; Velayudhan, M.P. Design, synthesis, antimicrobial and antioxidant activity of 3-formyl chromone hydrazone and their metal (II) complexes. Inorg. Chim. Acta 2018, 469, 87–97. [Google Scholar] [CrossRef]
- Wang, G.; Chen, M.; Wang, J.; Peng, Y.; Li, L.; Xie, Z.; Deng, B.; Chen, S.; Li, W. Synthesis, biological evaluation and molecular docking studies of chromone hydrazone derivatives as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2957–2961. [Google Scholar] [CrossRef] [PubMed]
- Shebl, M.; Adly, O.M.I.; Taha, A.; Elabd, N.N. Structural variety in copper (II) complexes of 3-formylchromone: Synthesis, spectral, thermal, molecular modeling and biological studies. J. Mol. Struct. 2017, 1147, 438–451. [Google Scholar] [CrossRef]
- Smart, B.E. Fluorine substituent effects (on bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Kirk, K.L. Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. J. Fluor. Chem. 2006, 127, 1013–1029. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Van Loveren, C. The antimicrobial action of fluoride and its role in caries inhibition. J. Dent. Res. 1990, 69, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.E. Antimicrobial actions of fluoride for oral bacteria. Can. J. Microbiol. 1995, 41, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Aust, S.D.; Morehouse, L.A.; Thomas, C.E. Role of metals in oxygen radical reactions. J. Free Radic. Biol. Med. 1985, 1, 3–25. [Google Scholar] [CrossRef]
- Turnlund, J.R.; Keyes, W.R.; Anderson, H.L.; Acord, L.L. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am. J. Clin. Nutr. 1989, 49, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Daniel, K.G.; Harbach, R.H.; Guida, W.C.; Dou, Q.P. Copper storage diseases: Menkes, Wilson’s, and cancer. Front. Biosci. 2004, 9, 2652–2662. [Google Scholar] [CrossRef] [PubMed]
- Goodman, V.L.; Brewer, G.J.; Merajver, S.D. Copper deficiency as an anti-cancer strategy. Endocr. Relat. Cancer 2004, 11, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łazarenkow, A.; Michalska, M.; Mirowski, M.; Słomiak, K.; Nawrot-Modranka, J. The effect of hydrazine derivatives of 3-formylchromones on angiogenic basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanoma cell line WM-115. Acta Biochim. Pol. 2017, 64, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Mirowski, M.; Kaplińska, K.; Kusztal, D.; Łazarenkow, A.; Nawrot-Modranka, J. Effect of phosphorohydrazone derivatives of chromone on fibrin polymerization in the presence of bFGF. Indian J. Biochem. Biophys. 2013, 50, 227–232. [Google Scholar] [PubMed]
- Łazarenkow, A.; Michalska, M.; Gorąca, A.; Mirowski, M.; Nawrot-Modranka, J.; Piechota-Polanczyk, A. The influence of chromone based hydrazones on lipid peroxidation and bFGF concentration in the HL-60 cell line. Acta Biochim. Pol. 2013, 60, 259–262. [Google Scholar] [PubMed]
- Nohara, A.; Umetani, T.; Sanno, Y. Studies on antianaphylactic agents—I: A facile synthesis of 4-oxo-4H-1-benzopyran-3-carboxaldehydes by Vilsmeier reagents. Tetrahedron 1974, 30, 3553–3561. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. A general definitions of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- CrysAlisPro, version 1.171.38.41q; Rigaku Oxford Diffraction: Yarnton, UK, 2015. Available online: https://www.rigaku.com/en/products/smc/crysalis (accessed on 21 June 2018).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- National Reference Center for Antimicrobial Susceptibility (KORLD). Available online: http://www.korld.edu.pl/spec_rekomendacje.php (accessed on 21 June 2018).
- Jost, L.M.; Kirkwood, J.M.; Whiteside, T.L. Improved short- and long-term XTT-based colorimetric cellular cytotoxicity assay for melanoma and other tumor cells. J. Immunol. Methods 1992, 147, 153–165. [Google Scholar] [CrossRef]
- Huang, H.S.; Chiub, H.F.; Yehc, P.F.; Yuana, C.L. Structure-based design and synthesis of regioisomeric disubstituted aminoanthraquinone derivatives as potential anticancer agents. Helv. Chim. Acta 2004, 87, 999–1005. [Google Scholar] [CrossRef]
- DELL Statistica (Data Analysis Software System). Available online: https://www.statsoft.pl (accessed on 16 August 2018).
Sample Availability: Samples of the compounds are available from the authors. |





| 1 | 3 | 5 | 8 | 10 | |
|---|---|---|---|---|---|
| Empirical formula | C16H10F2N2O2 | C17H11F3N2O2 | C34H22F2N4O6 H2O | C16H9F3N2O2 | C34H22F6N4O4 |
| Formula weight | 300.26 | 332.28 | 638.57 | 318.25 | 664.55 |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Triclinic | monoclinic |
| Space group | Pī | P21/c | P21/n | Pī | C2/c |
| a (Å) | 6.8791 (2) | 16.8965 (9) | 11.1731 (10) | 3.9211 (1) | 25.5373 (11) |
| b (Å) | 12.0848 (4) | 4.8728 (2) | 16.4967 (12) | 11.7020 (2) | 7.4105 (3) |
| c (Å) | 16.1485 (6) | 18.4032 (10) | 16.4583 (12) | 14.2192 (3) | 14.7383 (5) |
| α (°) | 94.432 (3) | 90 | 90 | 88.791 (2) | 90 |
| β (°) | 100.817 (3) | 105.343 (5) | 109.125 (9) | 87.938 (2) | 93.014 (3) |
| γ (°) | 103.431 (3) | 90 | 90 | 87.939(2) | 90 |
| V (Å3) | 1272.24 (8) | 1461.19 (13) | 2866.1 (4) | 651.47 (2) | 2785.28 (19) |
| Z | 4 | 4 | 4 | 2 | 4 |
| T (K) | 100 (1) | 290 (1) | 293 (1) | 100 (1) | 91 (1) |
| F (000) | 616 | 680 | 1320 | 324 | 1360 |
| Dx (g cm−3) | 1.568 | 1.510 | 1.480 | 1.622 | 1.585 |
| µ (mm−1) | 0.125 | 0.126 | 0.114 | 0.138 | 0.132 |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| θ range (°) | 3.11–30.0 | 3.75–30.0 | 3.13–30.0 | 3.32–30.0 | 3.12–30.0 |
| Measured reflections | 14, 205 | 18, 926 | 30, 676 | 8452 | 14, 914 |
| Unique reflections | 7388 | 4247 | 8335 | 3806 | 4067 |
| Observed reflections [I > 2σ (I)] | 5176 | 2004 | 3518 | 3359 | 2917 |
| Completeness to θmax (%) | 99.5 | 99.8 | 99.8 | 99.7 | 99.8 |
| Parameters/restraints | 405/0 | 249/93 | 441/0 | 212/0 | 244/0 |
| R [I > 2σ (I)] | 0.0481 | 0.0696 | 0.0607 | 0.0336 | 0.0518 |
| wR (all data) | 0.1372 | 0.2536 | 0.1905 | 0.1016 | 0.1434 |
| S | 1.006 | 1.030 | 1.011 | 1.062 | 1.058 |
| Δρmax (e Å−3) | 0.494 | 0.299 | 0.216 | 0.443 | 0.561 |
| Δρmin (e Å−3) | −0.278 | −0.365 | −0.214 | −0.194 | −0.490 |
| Comp. | H–Bond | D–H | H⋅⋅⋅A | D⋅⋅⋅A | D–H⋅⋅⋅A |
|---|---|---|---|---|---|
| 1 | N2–H2A⋅⋅⋅F4 | 0.90 (2) | 2.45 (2) | 2.937 (2) | 114 (2) |
| N2–H2A⋅⋅⋅O4i | 0.90 (2) | 2.28 (2) | 3.091 (2) | 150 (2) | |
| N4–H4A⋅⋅⋅O2i | 0.86 (2) | 2.15 (2) | 2.957 (2) | 158 (2) | |
| 3 | N2–H2A⋅⋅⋅O2i | 0.91 (3) | 2.00 (3) | 2.903 (3) | 172 (2) |
| 5 | N2–H2A⋅⋅⋅O6i | 0.83 (2) | 2.11 (2) | 2.910 (2) | 163 (2) |
| N4–H4A⋅⋅⋅O7 | 0.90 (2) | 1.91 (3) | 2.785 (2) | 162 (2) | |
| O7–H7A⋅⋅⋅O3 | 0.95 (3) | 1.83 (3) | 2.750 (2) | 162 (2) | |
| O7−H7A⋅⋅⋅N1 | 0.95 (3) | 2.50 (3) | 3.143 (2) | 125 (2) | |
| O7–H7B⋅⋅⋅O6ii | 0.87 (4) | 2.17 (4) | 2.929 (3) | 145 (3) | |
| O7–H7B⋅⋅⋅N3ii | 0.87 (4) | 2.42 (4) | 3.061 (3) | 131 (3) | |
| 8 | N2–H2A⋅⋅⋅O2i | 0.85 (2) | 2.10 (2) | 2.926 (1) | 163 (2) |
| 10 | N2–H2A⋅⋅⋅O2i | 0.90 (2) | 2.32 (2) | 3.150 (2) | 154 (2) |
| N2–H2A⋅⋅⋅F1 | 0.90 (2) | 2.27 (2) | 2.849 (2) | 122 (2) |
| Strain | 1 | 4 | 7 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|
| S. epidermidis | 100 µg/mL | 100 µg/mL | 100 µg/mL | 100 µg/mL | ||
| S. pneumoniae | 100 µg/mL | |||||
| S. pyogenes | 200 µg/mL | |||||
| S. aureus | 100 µg/mL | |||||
| N. meningitidis | 100 µg/mL * |
| Compound | IC50 [µmol/L] | The Range of Influence [%, Control = 100%] | ||
|---|---|---|---|---|
| L929 | EA.hy926 | L929 | EA.hy926 | |
| Cisplatin | 74.44 | 67.36 | 8.49–55.70 | 8.71–75.14 |
| 1 | 208.51 | 59.15 | 27.64–101.8 | 14.79–106.26 |
| 3 | 3714.50 | 975.09 | 55.91–96.98 | 37.40–101.33 |
| 4 | 1034.69 | 533.79 | 45.61–101.31 | 28.46–102.18 |
| 5 | 65.92 | 0.18 | 16.03–56.35 | 11.81–60.64 |
| 6 | 727.78 | 578.25 | 40.05–93.11 | 24.47–97.86 |
| 7 | 953.76 | 953.37 | 44.89–96.11 | 42.43–101.05 |
| 8 | 98.49 | 18.12 | 38.11–64.40 | 23.31–63.94 |
| 9 | 385.65 | 253.00 | 13.92–85.11 | 13.15–69.88 |
| 10 | 1207.48 | 645.48 | 48.62–73.47 | 31.21–94.69 |
| 11 | 35.01 | 0.04 | 10.30–75.02 | 8.58–53.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słomiak, K.; Łazarenkow, A.; Chęcińska, L.; Kusz, J.; Ochocki, J.; Nawrot-Modranka, J. Synthesis, Spectroscopic Analysis and Assessment of the Biological Activity of New Hydrazine and Hydrazide Derivatives of 3-Formylchromone. Molecules 2018, 23, 2067. https://doi.org/10.3390/molecules23082067
Słomiak K, Łazarenkow A, Chęcińska L, Kusz J, Ochocki J, Nawrot-Modranka J. Synthesis, Spectroscopic Analysis and Assessment of the Biological Activity of New Hydrazine and Hydrazide Derivatives of 3-Formylchromone. Molecules. 2018; 23(8):2067. https://doi.org/10.3390/molecules23082067
Chicago/Turabian StyleSłomiak, Krzysztof, Andrzej Łazarenkow, Lilianna Chęcińska, Joachim Kusz, Justyn Ochocki, and Jolanta Nawrot-Modranka. 2018. "Synthesis, Spectroscopic Analysis and Assessment of the Biological Activity of New Hydrazine and Hydrazide Derivatives of 3-Formylchromone" Molecules 23, no. 8: 2067. https://doi.org/10.3390/molecules23082067





