Synthesis of Novel Triazinoindole-Based Thiourea Hybrid: A Study on α-Glucosidase Inhibitors and Their Molecular Docking
Abstract
:1. Introduction
2. Results and Discussion
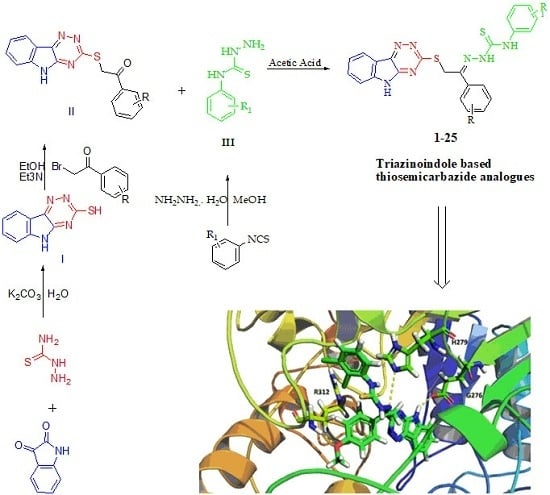
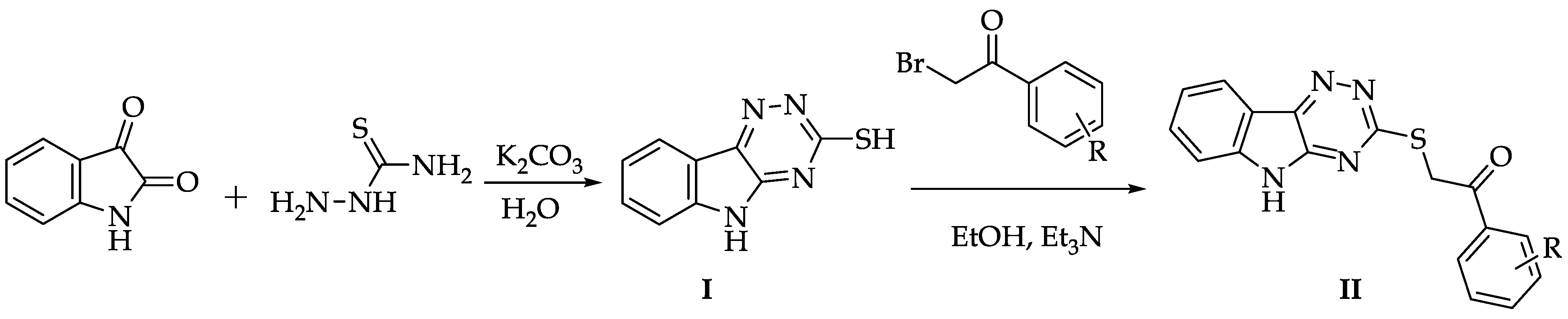
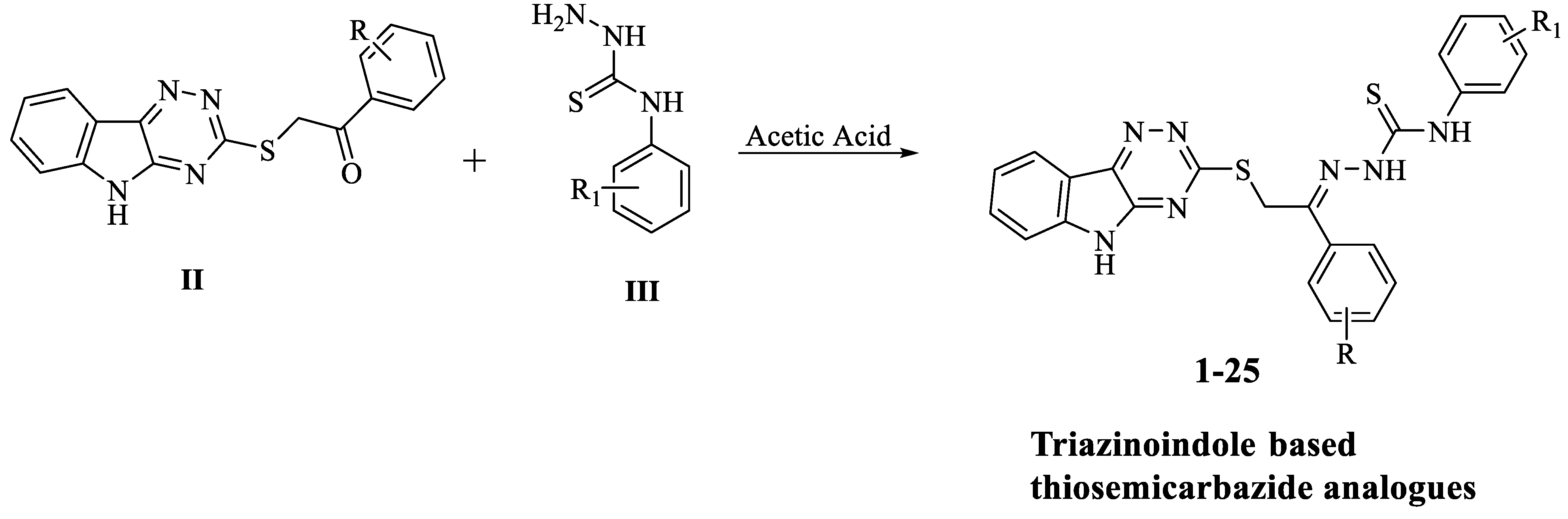
2.1. Chemistry
2.2. Biological Activity
2.3. Docking Study
3. Conclusions
4. Experiment
4.1. General Method for the Synthesis of Triazinoindole-Bearing Thiosemicarbazide Analogs (1–25)
4.2. α-Glucosidase Assay Protocol
- 70 μL of 50 mM phosphate buffer (pH 6.8);
- 10 μL (0.5 mM in methanol) test compounds; and
- 10 μL (0.057 units, Sigma Inc.) of enzyme solution in buffer.
4.3. Molecular Docking
Author Contributions
Funding
Conflicts of Interest
References
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderol B: A potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine 2011, 18, 1053. [Google Scholar] [CrossRef]
- Rother, K.I. Diabetes treatment—Bridging the divide. New Engl. J. Med. 2007, 356, 1499. [Google Scholar] [CrossRef] [PubMed]
- Casirola, D.M.; Ferraris, R.P. Alpha-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism 2006, 55, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; Group, S.N.T.R. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. J. Am. Med. Assoc. 2003, 290, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, R.; Tajima, N.; Iwamoto, Y.; Kashiwagi, A.; Shimamoto, K.; Kaku, K.; Group, V.P.S. Voglibose for prevention of type 2 diabetes mellitus: A randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009, 373, 1607–1614. [Google Scholar] [CrossRef]
- Monge, A.; Palop, J.; Ramierz, C.; Font, M.; Fernandez-Alveraz, E. New 5H-1,2,4-triazino[5,6-b]indole and aminoindole derivatives. Synthesis and studies as inhibitors of blood platelet aggregation, anti-hypertensive agents and thromboxane synthetase inhibitorsNouveaux dérivés 5H-1,2,4-triazino[5,6-b]indole et de l’aminoindole. Synthèse et évaluation comme inhibiteurs de l’agrégation plaquettaire, agents anti-hypertenseurs et inhibiteurs de la thromboxane synthétase. Eur. J. Med. Chem. 1991, 26, 179. [Google Scholar]
- Shelke, S.M.; Bhosale, S.H. Synthesis, antidepressant evaluation and QSAR studies of novel 2-(5H-[1,2,4]triazino[5,6-b]indol-3-ylthio)-N-(substituted phenyl)acetamides. Bioorg. Med. Chem. Lett. 2010, 20, 4661–4664. [Google Scholar] [CrossRef]
- Tomchin, A.B.; Uryupov, O.Y.; Zhukova, T.I.; Kuznetsova, T.A.; Kostycheva, M.V.; Smirnov, A.V. Thiourea and Thiosemicarbazide Derivatives: Structure, Transformations, and Pharmacological Activity. Part II. Antihypoxic Activity of 1,2,4-triazino[5,6-b]indole Derivatives. Pharmaceut. Chem. J. 1997, 31, 125–133. [Google Scholar] [CrossRef]
- Aswar, U.M.; Kalshetti, P.P.; Shelke, S.M.; Bhosale, S.H.; Bodhankar, S.L. Effect of newly synthesized 1,2,4-triazino[5,6-b]indole-3-thione derivatives on olfactory bulbectomy induced depression in rats. Asian Pac. J. Trop. Biomed. 2012, 2, 992. [Google Scholar] [CrossRef]
- Kgokong, J.L.; Smith, P.P.; Matsabisa, G.M. 1,2,4-Triazino-[5,6b]indole derivatives: Effects of the trifluoromethyl group on in vitro antimalarial activity. Bioorg. Med. Chem. 2005, 13, 2935–2942. [Google Scholar] [CrossRef]
- Gladych, J.M.Z.; Hunt, J.H.; Jack, D.; Haff, R.F.; Boyle, J.J.; Stewart, R.C.; Ferlanto, R.J. Inhibition of Rhinovirus by Isatin Thiosemicarbazone Analogues. Nature 1969, 221, 286. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.J.; Raupp, W.G.; Stanfield, F.J.; Haff, R.F.; Dick, E.C.; Alessio, D.D.; Dick, C.R. Progress in Rhinovirus Chenotherapy. Ann. N. Y. Acad. Sci. 1970, 173, 477. [Google Scholar] [CrossRef]
- Gwaltney, J.M. Rhinovirus inhibition by 3-substituted triazinoindoles. Proc. Soc. Exp. Biol. Med. 1970, 133, 1148. [Google Scholar] [CrossRef] [PubMed]
- Haff, R.F.; Flagg, W.B.; Gallo, J.J.; Hoover, J.R.E.; Miller, J.A.; Pinto, C.A.; Pagano, J.F. The in vitro antiviral activity of a triazinoindole (SK & F 40491). Proc. Soc. Exp. Biol. Med. 1972, 141, 475–478. [Google Scholar]
- Noreen, T.; Taha, M.; Imran, S.; Chigurpati, S.; Rahim, F.; Selvaraj, M.; Ismail, N.H.; Mohammad, J.I.; Ullah, H.; Javid, M.T.; et al. Synthesis of Alpha Amylase Inhibitors Based on Privileged Indole Scaffold. Bioorg. Chem. 2017, 72, 248–255. [Google Scholar] [CrossRef]
- Rahim, F.; Ali, M.; Ullah, S.; Rashid, U.; Ullah, H.; Taha, M.; Javed, M.T.; Rehman, W.; Abid, O.U.R.; Khan, A.A.; et al. Development of bis-Thiobarbiturates as Successful Urease Inhibitors and their Molecular Modeling Studies. Chin. Chem. Lett. 2016, 27, 693–697. [Google Scholar] [CrossRef]
- Taha, M.; Sultan, S.; Nuzar, H.A.; Rahim, F.; Imran, S.; Ismail, N.H.; Naz, H.; Ullah, H. Synthesis and biological evaluation of novel N-arylidenequinoline-3-carbohydrazides as potent β-glucuronidase inhibitors. Bioorg. Med. Chem. 2016, 24, 3696–3704. [Google Scholar] [CrossRef]
- Zaman, K.; Rahim, F.; Taha, M.; Ullah, H.; Wadood, A.; Nawaz, M.; Khan, F.; Wahab, Z.; Shah, S.A.A.; Rehman, A.; et al. Synthesis, in vitro urease inhibitory potential and molecular docking study of Benzimidazole analogues. Bioorg. Chem. 2019, 89, 103024. [Google Scholar] [CrossRef]
- Rashid, U.; Rahim, F.; Taha, M.; Arshad, M.; Ullah, H.; Mahmood, T.; Ali, M. Synthesis of 2-Acylated and Sulfonated 4-hydroxycoumarins: In vitro Urease Inhibition and Molecular Docking Studies. Bioorg. Chem. 2016, 66, 111–116. [Google Scholar] [CrossRef]
- Khan, K.M.; Rasheed, H.; Fatima, B.; Hayat, M.; Rahim, F.; Ullah, H.; Hameed, A.; Taha, M.; Tahir, A.; Perveen, S. Anti-Cancer Potential of Benzophenone-Bis-Schiff bases on Human Pancreatic Cancer Cell Line. J. Chem. Soc. Pak. 2016, 38, 954–958. [Google Scholar]
- Taha, M.; Ismail, N.H.; Imran, S.; Rahim, F.; Wadood, A.; Khan, H.; Ullah, H.; Salar, U.; Khan, K.M. Synthesis, β-Glucuronidase Inhibition and Molecular Docking Studies of Hybrid Bisindole-Thiosemicarbazides Analogs. Bioorg. Chem. 2016, 68, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Javid, M.T.; Ullah, H.; Wadood, A.; Taha, M.; Ashraf, M.; Aine, Q.U.; Khan, M.A.; Khan, F.; Mirza, S.; et al. Synthesis, Molecular Docking, Acetylcholinesterase and Butyrylcholinesterase Inhibitory Potential of Thiazole Analogs as New Inhibitors for Alzheimer Disease. Bioorg. Chem. 2015, 62, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Rahim, F.; Taha, M.; Hussain, R.; Wadood, A.; Nawaz, M.; Wahab, Z.; Kanwal; Khan, K.M. Synthesis, In vitro α-Glucosidase Inhibitory Potential and Molecular Docking Studies of 2-Amino-1,3,4-Oxadiazole Derivatives. Med. Chem. 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Taha, M.; Wadood, A.; Javid, M.T.; Rehman, W.; Nawaz, M.; Ashraf, M.; Ali, M.; Sajid, M.; et al. Synthesis and in vitro Acetylcholinesterase and Butyrylcholinesterase Inhibitory Potential of Hydrazide based Schiff Bases. Bioorg. Chem. 2016, 68, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Taha, M.; Ullah, H.; Wadood, A.; Selvaraj, M.; Rab, A.; Sajid, M.; Shah, S.A.A.; Uddin, N.; Gollapalli, M. Synthesis of new arylhydrazide bearing Schiff bases/thiazolidinone: α-amylase, urease activities and their molecular docking studies. Bioorg. Chem. 2019, 91, 103112. [Google Scholar] [CrossRef]
- Gollapalli, M.; Taha, M.; Ullah, H.; Nawaz, M.; Al Muqarrabun, L.M.R.; Rahim, F.; Qureshi, F.; Mosaddik, A.; Ahmat, N.; Khan, K.M. Synthesis of Bis-indolylmethane sulfonohydrazides derivatives as potent α-Glucosidase inhibitors. Bioorg. Chem. 2018, 80, 112–120. [Google Scholar] [CrossRef]
- Ullah, H.; Rahim, F.; Taha, M.; Uddin, I.; Wadood, A.; Shah, S.A.A.; Farooq, R.K.; Nawaz, M.; Wahab, Z.; Khan, K.M. Synthesis, Molecular docking study and in vitro Thymidine Phosphorylase Inhibitory Potential of Oxadiazole Derivatives. Bioorg. Chem. 2018, 78, 58–67. [Google Scholar] [CrossRef]
- Taha, M.; Ullah, H.; Al Muqarrabun, L.M.R.; Khan, M.N.; Rahim, F.; Ahmat, N.; Javid, M.T.; Ali, M.; Khan, K.M. Bisindolylmethane thiosemicarbazides as potential inhibitors of urease: Synthesis and molecular modeling studies. Bioorg. Med. Chem. 2018, 26, 152–160. [Google Scholar] [CrossRef]
- Taha, M.; Ullah, H.; Al Muqarrabun, L.M.R.; Khan, M.N.; Rahim, F.; Ahmat, N.; Ali, M.; Perveen, S. Synthesis of bis-indolylmethanes as new potential inhibitors of β-glucuronidase and their molecular docking studies. Eur. J. Med. Chem. 2018, 143, 1757–1767. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, K.; Ullah, H.; Wadood, A.; Taha, M.; Rehman, A.; din, I.U.; Ashraf, M.; Shaukat, A.; Rehman, W.; et al. Triazinoindole analogs as potent inhibitors of α-glucosidase: Synthesis, biological evaluation and molecular docking studies. Bioorg. Chem. 2015, 58, 81–87. [Google Scholar] [CrossRef]
- Imran, S.; Taha, M.; Ismail, N.H.; Kashif, S.M.; Jamil, W.; Hariono, M.; Yusuf, M.; Wahab, H.; Imran, S. Synthesis of novel flavone hydrazones: In-vitro evaluation of α-glucosidase inhibition, QSAR analysis and docking studies. Eur. J. Med. Chem. 2015, 105, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Javid, M.T.; Wadood, A.; Taha, M.; Ashraf, M.; Shaukat, A.; Junaid, M.; Hussain, S.; Rehman, W.; et al. Synthesis, in vitro evaluation and molecular docking studies of thiazole derivatives as new inhibitors of α-glucosidase. Bioorg. Chem. 2015, 62, 15–21. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |






| S. No. | R | R1 | IC50 (μM) |
|---|---|---|---|
| 1 | 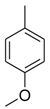 |  | 1.30 ± 0.05 |
| 2 |  | 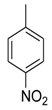 | 2.60 ± 0.05 |
| 3 | 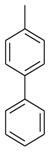 |  | 7.5 ± 0.20 |
| 4 |  |  | 12.80 ± 0.30 |
| 5 |  |  | 5.20 ± 0.30 |
| 6 |  | 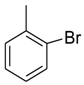 | 25.3 ± 0.50 |
| 7 |  |  | 33.30 ± 0.60 |
| 8 |  |  | 17.5 ± 0.30 |
| 9 | 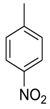 | 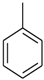 | 6.80 ± 0.10 |
| 10 |  |  | 35.80 ± 0.80 |
| 11 |  |  | 26.80 ± 0.70 |
| 12 |  |  | 8.30 ± 0.20 |
| 13 |  | 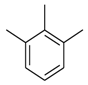 | 23.30 ± 0.50 |
| 14 |  |  | 7.5 ± 0.20 |
| 15 |  |  | 5.80 ± 0.20 |
| 16 |  | 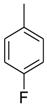 | 1.8 ± 0.20 |
| 17 | 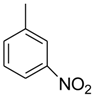 |  | 8.80 ± 0.20 |
| 18 |  | 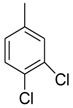 | 2.30 ± 0.05 |
| 19 |  |  | 2.30 ± 0.05 |
| 20 |  |  | 7.50 ± 0.20 |
| 21 |  |  | 4.80 ± 0.20 |
| 22 |  | 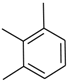 | 5.90 ± 0.10 |
| 23 |  | 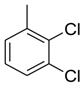 | 1.30 ± 0.01 |
| 24 |  |  | 15.3 ± 0.30 |
| 25 |  |  | 9.30 ± 0.30 |
| Acarbose | 38.60 ± 0.20 | ||
| Compounds | Chemscore (kJ/mol) | IC50 (μM ± SD) |
|---|---|---|
| 1 | −89.3 | 1.30 ± 0.05 |
| 16 | −74.5 | 1.80 ± 0.20 |
| 23 | −87.7 | 1.30 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, M.; Alshamrani, F.J.; Rahim, F.; Hayat, S.; Ullah, H.; Zaman, K.; Imran, S.; Khan, K.M.; Naz, F. Synthesis of Novel Triazinoindole-Based Thiourea Hybrid: A Study on α-Glucosidase Inhibitors and Their Molecular Docking. Molecules 2019, 24, 3819. https://doi.org/10.3390/molecules24213819
Taha M, Alshamrani FJ, Rahim F, Hayat S, Ullah H, Zaman K, Imran S, Khan KM, Naz F. Synthesis of Novel Triazinoindole-Based Thiourea Hybrid: A Study on α-Glucosidase Inhibitors and Their Molecular Docking. Molecules. 2019; 24(21):3819. https://doi.org/10.3390/molecules24213819
Chicago/Turabian StyleTaha, Muhammad, Foziah J. Alshamrani, Fazal Rahim, Shawkat Hayat, Hayat Ullah, Khalid Zaman, Syahrul Imran, Khalid Mohammed Khan, and Farzana Naz. 2019. "Synthesis of Novel Triazinoindole-Based Thiourea Hybrid: A Study on α-Glucosidase Inhibitors and Their Molecular Docking" Molecules 24, no. 21: 3819. https://doi.org/10.3390/molecules24213819