Glutathione Peroxidase-like Activities of Oxygen-Containing Diselenides
Abstract
:Introduction
Results and Discussion
Conclusions
Acknowledgements
References and Notes
- Reviews: Stadtman, T. C. J. Biol. Chem. 1991, 266, 16257–16260. [PubMed]Ursini, F. Oxidative Processes and Antioxidants; Paoletti, R., Ed.; Raven Press: New York, 1994; pp. 25–31. [Google Scholar]
- Rotruck, J. T.; Pope, A. L.; Ganther, H. E.; Swanson, A. B.; Hafeman, D. G.; Hoekstra, W. G. Science 1973, 179, 588–590. [PubMed]Flohé, L.; Gunzler, W. A.; Schock, H. H. FEBS Lett. 1973, 32, 132–134.
- Berry, M. J.; Banu, L.; Larsen, P. R. Nature 1991, 349, 438–440. [PubMed]Boyington, J. C.; Gladyshev, V. N.; Khangulov, S. V.; Stadtman, T. C.; Sun, P. D. Science 1997, 275, 1305–1308. [PubMed]
- Ladenstein, R.; Epp, O.; Bartels, K.; Jones, A.; Huber, R.; Wendel, A. J. Mol. Biol. 1979, 134, 199–218. [CrossRef] Epp, O.; Ladenstein, R.; Wendel, A. Eur. J. Biochem. 1983, 133, 51–69.
- Reich, H. J.; Jasperse, C. P. J. Am. Chem. Soc. 1987, 109, 5549–5551. [CrossRef] Wilson, S. R.; Zucker, P. A.; Huang, R.-R. C.; Spector, A. J. Am. Chem. Soc. 1989, 111, 5936–5939. [CrossRef] Iwaoka, M.; Tomoda, S. J. Am. Chem. Soc. 1994, 116, 2557–2561. [CrossRef] Engman, L.; Andersson, C.; Morgenstern, R.; Cotgreave, I. A.; Andersson, C.-M.; Hallberg, A. Tetrahedron 1994, 50, 2929–2938. Chaudière, J.; Yadan, J.-C.; Erdelmeier, I.; Tailhan-Lomont, C.; Moutet, M. Oxidative Processes and Antioxidants; Paoletti, R., Ed.; Raven Press: New York, 1994; pp. 165–184. [Google Scholar]
- Chaudière, J.; Moutet, M.; d’Alessio, P. C. R. Soc. Biol. 1995, 189, 861–882. Sies, H. Free Rad. Biol. Med. 1993, 14, 313–323.
- Aumann, K.-D.; Bedorf, N.; Brigelius-Flohé, R.; Schomburg, D.; Flohé, L. Biomed. Environ. Sci. 1997, 10, 136–155.
- Hargittai, I.; Rozsondai, B. The Chemistry of Organic Selenium and Tellurium Compounds; Patai, S., Ed.; Wiley: Chichester, U.K., 1986; Vol. 1, pp. 63–155. [Google Scholar]
- Galet, V.; Bernier, J.; Henichart, J.; Lesieur, D.; Abadie, C.; Rochette, L.; Lindenbaum, A.; Chalas, J.; Renaud de la Faverie, J.; Pfeiffer, B.; Renard, P. J. Med. Chem. 1994, 37, 2903–2911. [CrossRef] Mlochowski, J.; Giurg, M.; Kubicz, E.; Said, S. B. Synth. Commun 1996, 26, 291–300. Back, T. G.; Dyck, B. P. J. Am. Chem. Soc. 1997, 119, 2079–2083.
- Compounds 1–7: Wirth, T.; Fragale, G. Chem. Eur. J. 1997, 3, 1894–1902. Compounds 8: Uemura, S.; Takahashi, H.; Ohe, K.; Sugita, N. J. Organomet. Chem. 1989, 361, 63–72.
- Wirth, T.; Fragale, G.; Spichty, M. J. Am. Chem. Soc. 1998, 120, 3376–3381. [CrossRef]
- Paglia, D. E.; Valentine, W. N. J. Lab. Clin. Med. 1967, 70, 158–169.
- Samples Availability: Available from the author.
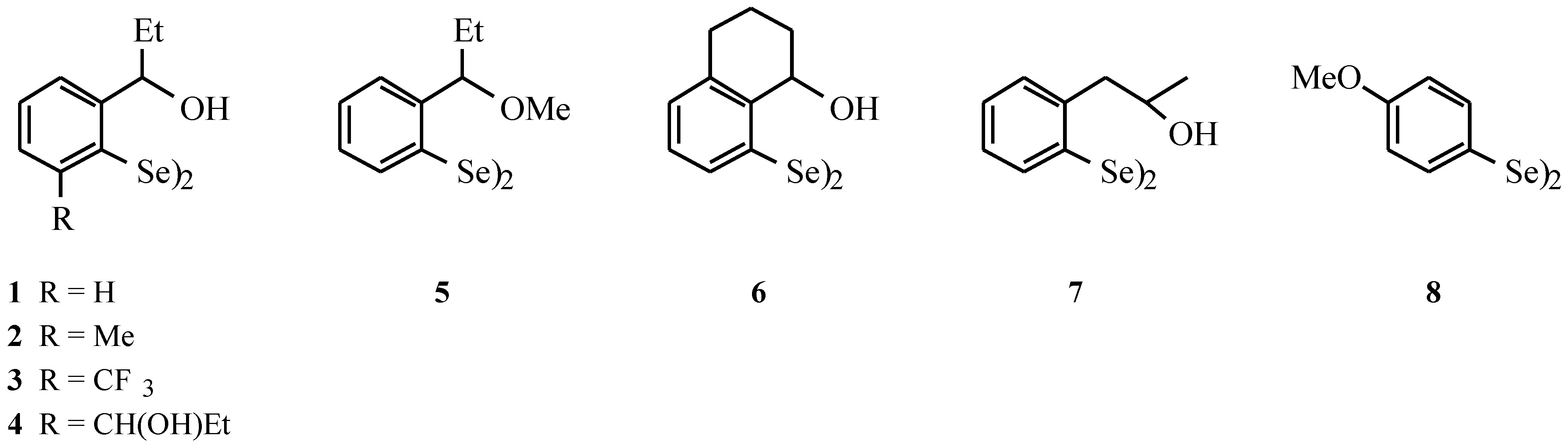

| Diselenide | GPx activities (20 µM Se-Equivalents) [nmol of NADPH•min–1] 100 µ M H2O2 | GPx activities (20 µM Se-Equivalents) [nmol of NADPH•min–1] 200 µ M t-BuOOH | GOx activities (20 µM Se-Equivalents) [nmol of NADPH•min–1] |
|---|---|---|---|
| 1 | 26.6 | 13.9 | 1.1 |
| 2 | 20.2 | 13.0 | 0.3 |
| 3 | 8.7 | 4.7 | 0 a) |
| 4 | 18.8 | 9.5 | 0.3 |
| 5 | 14.5 | 7.8 | 0.2 |
| 6 | 27.5 | 14.8 | 1.0 |
| 7 | 17.9 | 11.5 | 0.4 |
| 8 | 30.2 | 17.7 | 0.9 |
© 1998 MDPI. All rights reserved. Molecules http://www.mdpi.org/molecules/
Share and Cite
Wirth, T. Glutathione Peroxidase-like Activities of Oxygen-Containing Diselenides. Molecules 1998, 3, 164-166. https://doi.org/10.3390/30700164
Wirth T. Glutathione Peroxidase-like Activities of Oxygen-Containing Diselenides. Molecules. 1998; 3(7):164-166. https://doi.org/10.3390/30700164
Chicago/Turabian StyleWirth, Thomas. 1998. "Glutathione Peroxidase-like Activities of Oxygen-Containing Diselenides" Molecules 3, no. 7: 164-166. https://doi.org/10.3390/30700164




