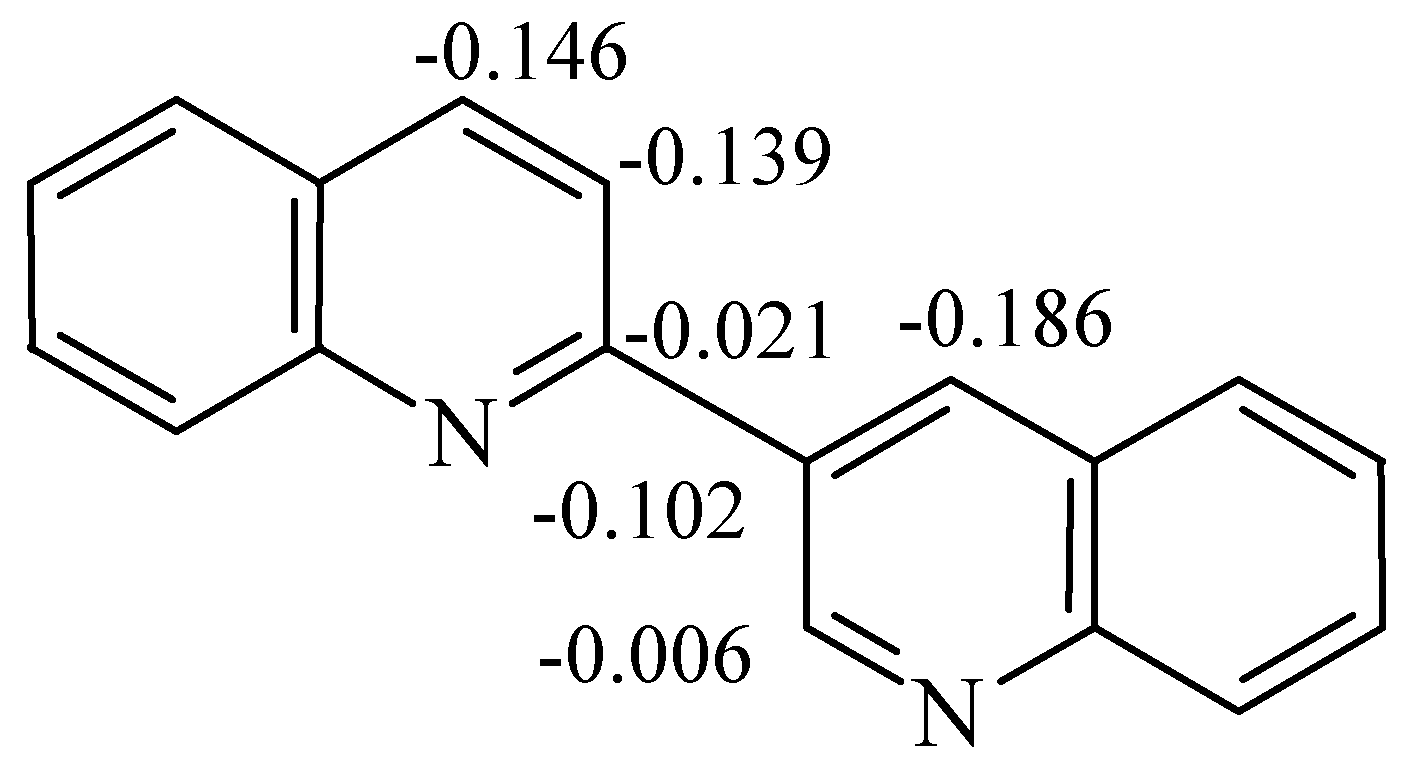
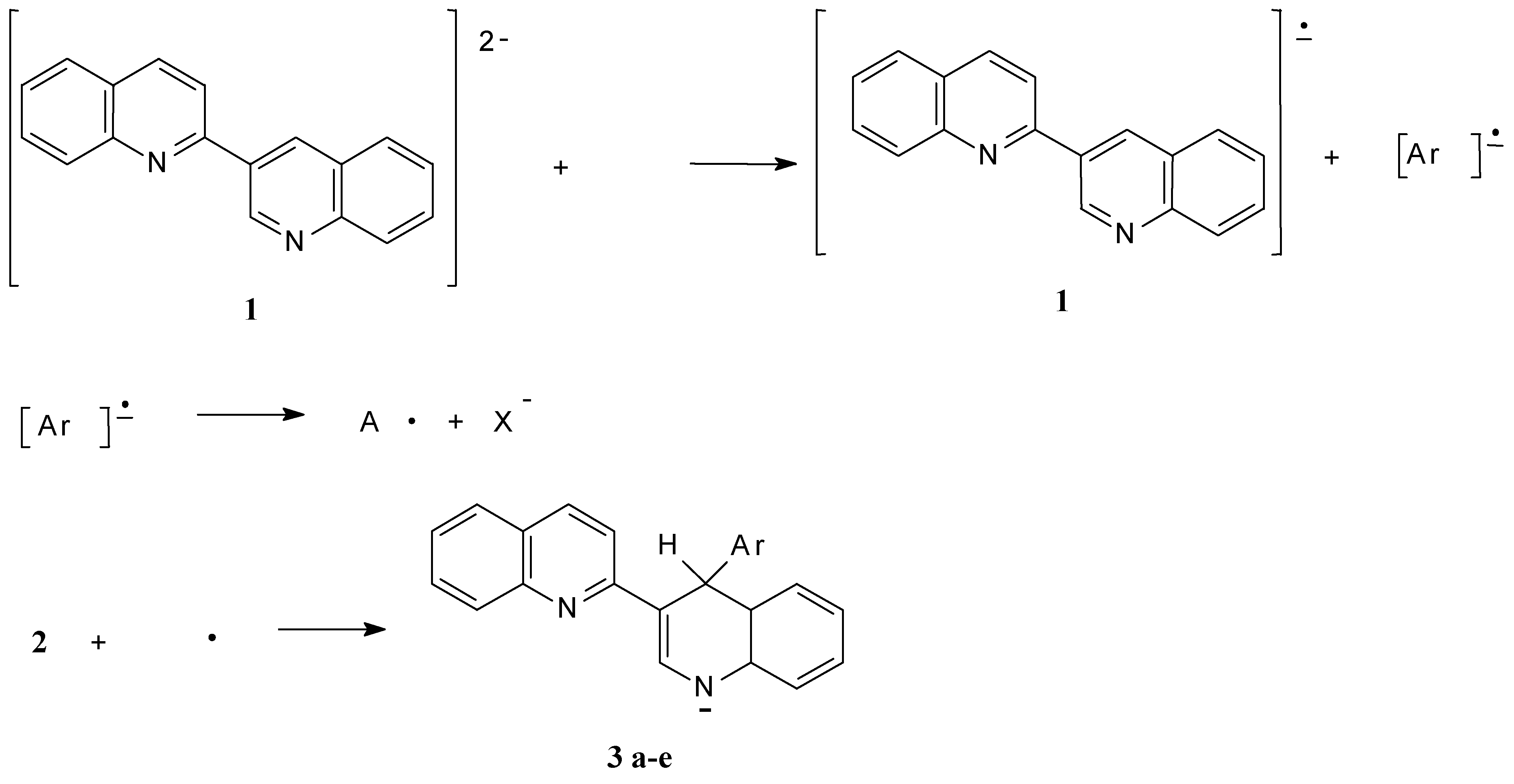
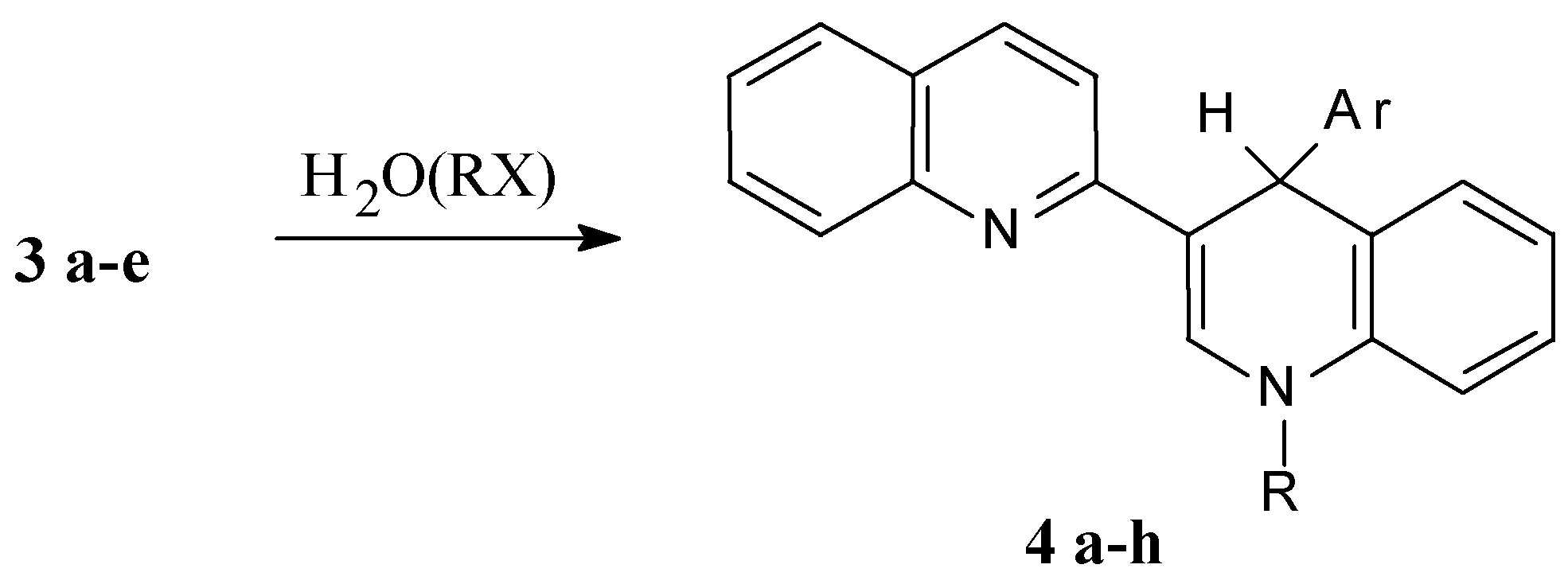
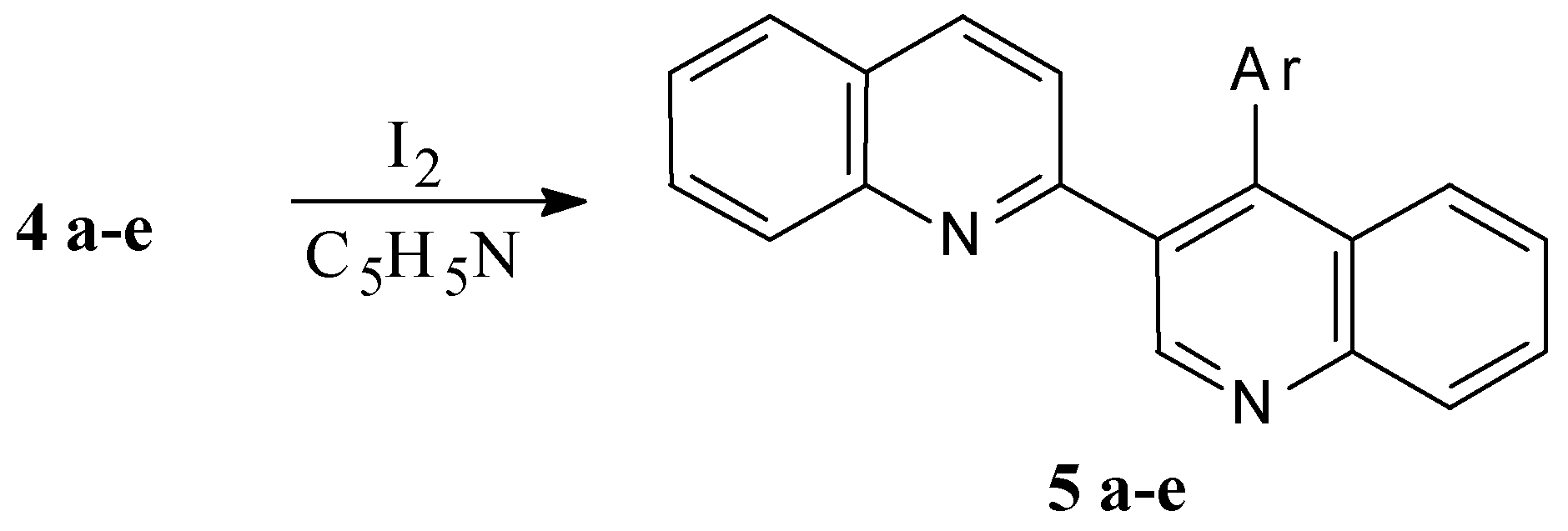
Mass-spectra were registered on a Varian MAT 331 A mass-spectrometer, NMR spectra at Bruker WP-200 NMR spectrometer. The reactions were monitored by TLC on Silufol UV-254 plates, eluent ethyl acetate–hexane 2:1. THF was purified by distillation over LiAlH4 and ketine radical, and 2,3’-biquinolyl was recrystallised from benzene and sublimed.
Syntheses of the compounds
4’-Phenyl-1’,4’-dihydro-2,3’-biquinolyl (4 a, C24H18N2)
A solution of 0.64 g (2.5 mmol) of 2,3’-biquinolyl and 0.05 g (7 mmol) of powdered lithium in 10 mL of THF is stirred for 3 h at ambient temperature under an Ar blanket. After that the solution of 0.71 g (4.5 mmol) bromobenzene in 4 mL THF is added dropwise. The reaction mixture is stirred for 1 hour at ambient temperature and refluxed for 1 h. After that 40 mL of water is added and the reaction mixture is extracted with benzene (3 × 30 mL). The organic solution is evaporated and the resulting yellow oil is crystallized on benzene addition. Yield 0.65 g (78%), m.p. 212-213 °C (bnz.). 1H NMR spectrum (CDCl3): 5.73 (1H, s, 4’-H); 6.36 (1H, d, JNH-2'H= 5.5 Hz, NH); 6.70 (1H, d, J7'8' = 8.10 Hz, 8'-H); 6.88 (1H, d. d, J5'6' =7.64, J6'7'= 7.82 Hz, 6'-H); 7.04 (1H, d. d, J6'7' = 7.82, J7'8' =8.12 Hz, 7'-H); 7.2 (3H, m, 3’’-H, 4’’-H, 5’’-H); 7.23 (1H, d, J5'6' =7.64 Hz, 5'-H); 7.34 (1H, d. d, J56 = 8.02, J67 = 7.51 Hz, 6-H); 7.38 (1H, d, J34 = 8.91 Hz, 3-H); 7.46 (2H, d, J= 7,1 Hz, 2’’-H, 6’’-H); 7.61 (1H, d. d, J67=7.51, J78 =8.61 Hz, 7-H); 7.62 (1H, d, J56 = 8.02 Hz, 5-H); 7.77 (1H, d, JNH-2'H=5.5 Hz, 2'-H); 7.86 (1H, d, J34 = 8.91 Hz, 4-H); 7.98 (1H, d, J78 =8.61 Hz, 8-H); determined, % C 86.28; H 5.29; N 8.43. C24H18N2, calculated, % C 86.19; H 5.43; N 8.38.
4’-(1-Naphthyl)-1’,4’-dihydro-2,3’-biquinolyl (4 b, C28H20N2)
Was obtained as above from 0.64 g (2.5 mmol) 2,3’-biquinolyl, 0.05 g (7 mmol) Li and 0.72 g (3.5 mmol) 1-bromonaphthalene. Yield 0.7 g (73%), m.p. 196-197 °C (bnz). 1H NMR spectrum (CDCl3): 6.38 (1H, d, JNH-2'H= 5.7 Hz, NH); 6.64 (1H, s, 4’-H); 6.72 (1H, d. d, J5'6' =7.72, J6'7'= 7.92 Hz, 6'-H); 6.74 (1H, d, J7'8' = 8.06 Hz, 8'-H); 7.01 (1H, d. d, J6'7' = 7.92, J7'8' =8.06 Hz, 7'-H); 7.23 (1H, d, J5'6' =7.72 Hz, 5'-H); 7.27-7.33 (3H, m, 6’-H, 3’’-H, 6’’-H); 7.49 (1H, d, J34 = 8.83 Hz, 3-H); 7.51-7.8 (7H, m, arom.); 7.81 (1H, d, J34 = 8.83 Hz, 4-H); 7.86 (1H, d, JNH-2'H=5.7 Hz, 2'-H); 9.01 (1H, d, J = 8.54, 8’’-H or 8-H); determined, % C 87.71; H 5.12; N 7.17. C28H20N2. Calculated, % C 87.46; H 5.25; N 7.29.
4’-(2-Pyridyl)-1’,4’-dihydro-2,3’-biquinolyl (4 c, C23H17N3)
As 4 a from 0.64 g (2.5 mmol) 2,3’-biquinolyl, 0.05 g (7 mmol) Li and 0.34 g (3.5 mmol) 2-fluoropyridine. Yield 0.56 g (67%), m.p. 219-220° C (bnz). 1H NMR spectrum (CDCl3): 5.88 (1H, s, 4’-H); 6.60 (1H, d, JNH-2'H= 5.3 Hz, NH); 6.72 (1H, d, J7'8' = 7.94 Hz, 8'-H); 6.88 (1H, d. d, J5'6' =7.68, J6'7'= 7.79 Hz, 6'-H); 6.98 (1H, m, 5’’-H); 7.07 (1H, d. d, J6'7' = 7.79, J7'8' =7.94 Hz, 7'-H); 7.34 (1H, d, J5'6' =7.68 1.63 Hz, 5'-H); 7.38 (1H, d. d., J56 = 7.98, J67 = 7.62 Hz, 6-H); 7.46 (1H, d, J34 = 8.96 Hz, 3-H); 7.5 (2H, m, 3’’-H, 4’’-H); 7.57 (1H, d. d., J67=7.62, J78 =8.46 Hz, 7-H); 7.62 (1H, d, J56 = 7.98 Hz, 5-H); 7.88 (1H, d, J34 = 8.96 Hz, 4-H); 7.90 (1H, d, J78 =8.46, J68 = 1.1 Hz, 8-H); 7.92 (1H, d, JNH-2'H=5.3 Hz, 2'-H); 8.51 (1H, d. d., J5’’6’’ = 4.7, J4’’6’’ = 1.7 Hz, 6’’-H); determined, % C 82.64; H 4.98; N 12.38. C23H17N3. Calculated, % C 82.36; H 5.11; N 12.53.
4’-(1-Methyl-2-benzimidazolyl)-1’,4’-dihydro-2,3’-biquinolyl (4 d, C26H20N4)
As 4 a from 0.64 g (2.5 mmol) 2,3’-biquinolyl, 0.05 g (7 mmol) Li and 0.58 g (3.5 mmol) 1-methyl-2-chlorobenzimidazole. Yield 0.66 g (68%), m.p. 266-267 °C (bnz). 1H NMR spectrum (CDCl3): 4.44 (3H, s, Me); 6.36 (1H, s, 4’-H); 6.70 (1H, d, J7'8' = 8.05 Hz, 8'-H); 6.81 (1H, d. d., J5'6' =7.68, J6'7' = 7.34 Hz, 6'-H); 6.92 (1H, d. d., J6'7' = 7.34, J7'8' =8.05, 7'-H); 7.09 (1H, d, J5'6' =7.68 Hz, 5'-H); 7.13 (1H, d. t., J4’’5’’ = 8.55, J5’’6’’ = 7.38, J5’’7’’ = 1.63 Hz, 5’’-H); 7.21 (1H, d. t., J5’’6’’ = 7.38, J6’’7’’ = 7.67, J4’’6’’ = 2.35 Hz, 6’’-H); 7.29 (1H, d. d., J56 = 8.04, J67 = 7.31 Hz, 6-H); 7.38 (1H, d. d., J6’’7’' =7.67, J5’’7’' = 1.63 Hz, 7’'-H); 7.41 (1H, d, JNH-2'H=5.49 Hz, 2'-H); 7.43 (1H, d, J34 = 9.14 Hz, 3-H); 7.53 (1H, d. d., J67=7.31, J78 =8.04 Hz, 7-H); 7.59 (1H, d. d., J4’'5’' =8.55, J4’'6’' = 2.35 Hz, 4’'-H); 7.6 (1H, d., J56 = 8.04 Hz, 5-H); 7.78 (1H, d, J78 =8.04 Hz, 8-H); 7.80 (1H, d, J34 = 9.14 Hz, 4-H); 10.39 (1H, d, JNH-2'H= 5.49 Hz, NH); determined, % C 80.63, H 5.11, N 14.26, C26H20N4. Calculated C 80.39, H 5.19, N 14.42.
4’-(1-iso-Propyl-2-benzimidazolyl)-1’,4’-dihydro-2,3’-biquinolyl (4 e, C28H24N4)
As 4 a from 0.64 g (2.5 mmol) 2,3’-biquinolyl, 0.05 g (7 mmol) Li and 0.68 g (3.5 mmol) 1-iso-propyl-2-chlorobenzimidazol. Yield 0.67 g (64%), m.p. 206-208° C (bnz). ). 1H NMR spectrum (CDCl3): 1.73 (3H, d., J= 6.95, Me); 1.85 (3H, d, J= 6.95, Me); 5.86 (1H, m, CH); 6.58 (1H, s, 4’-H); 6.81 (1H, d, J7'8' = 7.98, Hz, 8'-H); 6.85 (1H, d. d., J5'6' =7.58, J6'7' = 7.42 Hz, 6'-H); 7.0 (1H, d. d., J6'7' = 7.42, J7'8' =7.98 Hz, 7'-H); 7.1 (2H, m, 5’’-H, 6’’H); 7.2 (1H, d, J5'6' = 7.58 Hz, 5'-H); 7.30 (1H, d. d., J56 = 8.08, J67 = 7.41 Hz, 6-H); 7.48 (1H, d, J34 = 9.05 Hz, 3-H); 7.5 (1H, d, JNH-2'H=5.49 Hz, 2'-H); 7.54-7.61 (4H, m, 5-H, 7-H, 4’'-H, 7’'-H); 7.79 (1H, d, J34 = 9.05 Hz, 4-H); 7.88 (1H, d, J78 =8.34 Hz, 8-H); 10.33 (1H, d, JNH-2'H= 5.49 Hz, NH); determined, % C 80.95; H 5.68; N 13.37. C28H24N4. Calculated, % C 80.74; H 5.81; N 13.45.
1’-Methyl-4’-phenyl-1’,4’-dihydro-2,3’-biquinolyl (4 f, C25H20N2)
The solution of 0.64 g (2.5 mmol) 2,3’-biquinolyl and 0.05 g (7 mmol) of powdered Li in 10 mL THF is stirred under an Ar blanket at ambient temperature for 3 h, then a solution of 0.71 g (4.5 mmol) of bromobenzene in 4 mL THF is added dropwise. The reaction mixture is stirred for 1 h at room temperature, than refluxed for 1 h. The mixture is cooled to room temperature 0.71 g MeI in 2 mL THF is added and mixture is stirred for 1 h. The mixture is poured into 50 ml of water and extracted with benzene (3 × 30 mL). The organic solution is evaporated and resulting yellow oil is crystallized from hexane addition. Yield 0.65 g (75%), m.p. 173-174 °C (EtOH). 1H NMR spectrum (CDCl3): 3.38 (3H, s, Me); 5.68 (1H, s, 4’-H); 6.84 (1H, d, J7'8' = 8.12 Hz, 8'-H); 6.89 (1H, d. d., J5'6' =7.61, J6'7'= 7.76 Hz, 6'-H); 7.12 (1H, d. d., J6'7' = 7.76, J7'8' =8.12 Hz, 7'-H); 7.19 (3H, m, 3’’-H, 4’’-H, 5’’-H); 7.27 (1H, d, J5'6' =7.61 Hz, 5'-H); 7.35 (1H, d. d., J56 = 7.99, J67 = 7.56 Hz, 6-H); 7.42 (1H, d, J34 = 9.01 Hz, 3-H); 7.48 (2H, d, J= 7.13 Hz, 2’’-H, 6’’-H); 7.64 (1H, d. d., J67=7.56, J78 =8.53 Hz, 7-H); 7.67 (1H, d, J56 = 7.99 Hz, 5-H); 7.81 (1H, s, 2'-H); 7.91 (1H, d, J34 = 9.01 Hz, 4-H); 7.99 (1H, d, J78 =8.53 Hz, 8-H); determined, %,: C 86.44; H 5.67; N 7.89. .C25H20N2. Calculated, % C 86.18; H 5.79; N 8.03.
1’-Methyl-4’-(1-naphthyl)-1’,4’-dihydro-2,3’-biquinolyl (4 g, C29H22N2)
As 4 f from 0.64 g (2.5 mmol) 2,3’-biquinolyl, 0.05 g (7 mmol) Li, 0.72 g (3.5 mmol) 1-bromonaphthalene and 0.71 g (5 mmol) MeI. Yield 0.73 g (73%), m.p. 151-153 °C (benzene-hexane). 1H NMR spectrum (CDCl3): 3.51 (3H, s, Me); 6.59 (1H, s, 4’-H); 6.77 (1H, d. d., J5'6' =7.78, J6'7'= 7.62 Hz, 6'-H); 6.88 (1H, d, J7'8' = 8.04 Hz, 8'-H); 7.1 (1H, d. d., J6'7' = 7.62, J7'8' =8.04 Hz, 7'-H); 7.23-7.28 (4H, m, 5’-H, 6’-H, 3’’-H, 6’’-Í); 7.49 (1H, d, J34 = 8.79 Hz, 3-H); 7.51-7.8 (7H, m, arom.); 7.81 (1H, d, J34 = 8.88 Hz, 4-H); 7.83 (1H, s, 2'-H); 8.99 (1H, d, J = 8.54, 8’’-H or 8-H); determined, %,: C 87.64; H 5.47; N 6.89. C29H22N2. Calculated, %: C 87.41; H 5.56; N 7.03.
1’-Benzyl-4’-(1’-naphthyl)-1’,4’-dihydro-2,3’-biquinolyl (4 h, C35H26N2)
As 4 f from 0.64 g (2.5 mmol) 2,3’-biquinolyl, 0.05 g (7 mmol) Li, 0.72 g (3.5 mmol) 1-bromonaphthalene and 0.44 g (3.5 mmol) PhCH2Cl. Yield 0.85 g (72%), m.p. 143-144 °C (EtOH). 1H NMR spectrum (CDCl3): 5.1 (2H, d, J = 4.38 Hz, CH2); 6.69 (1H, s, 4’-H); 6.77 (1H, d. d., J5'6' =7.58, J6'7'= 7.36 Hz, 6'-H); 6.78 (1H, d, J7'8' = 7.82 Hz, 8'-H); 6.95 (1H, d. d., J6'7' = 7.36, J7'8' = 7.82 Hz, 7'-H); 7.04 (1H, d, J5'6' =7.58 Hz, 5'-H); 7.29-7.59 (8H, m, arom.); 7.41 (1H, d, J34 = 8.91 Hz, 3-H); 7.42-7.8 (7H, m, arom.); 7.81 (1H, d, J34 = 8.91 Hz, 4-H); 7.93 (1H, s, 2'-H); 9.03 (1H, d, J = 8.56, 8’’-H or 8-H); determined, %,: C 88.75; H 5.44; N 5.81 C35H26N2. Calculated, %: C 88.58; H 5.52; N 5.90.
4’-Phenyl -2,3’-biquinolyl (5 a, C24H16N2)
A solution of 0.42 g (1.25 mmol) 4’-phenyl-1’,4’-dihydro-2,3’-biquinolyl (4 a) and 0.33 g I2 in 5 mL pyridine is refluxed for 10 min. The reaction mixture is poured into 100 mL of water containing 1 g Na2S2O3 and 0.5 g NaOH, the white precipitate formed is filtered off and washed with water (3 × 30 mL). Yield 0.4 g (95%), m.p. 133-134 °C (bnz). 1H NMR spectrum (CDCl3): 6.97 (1H, d, J34 = 8.54 Hz, 3-H); 7.35 (5H, m, Ph); 7.52 (1H, d. d., J5'6' =8.11, J6'7'= 6.95 Hz, 6'-H); 7.56 (1H, d. d., J56 = 8.04, J67 = 7.01 Hz, 6-H); 7.72 (1H, d. d., J6'7' = 6.95, J7'8' =8.38 Hz, 7'-H); 7.74 (1H, d. d., J67=7.01, J78 =8.31 Hz, 7-H); 7.76 (1H, d., J56 = 8.04 Hz, 5-H); 7.79 (1H, d, J5'6' = 8.11 Hz, 5'-H); 7.84 (1H, d, J34 = 8.54 Hz, 4-H); 8.19 (1H, d, J7'8' = 8.38 Hz, 8'-H); 8.25 (1H, d, J78 =8.31 Hz, 8-H); 9.40 (1H, s, 2'-H); mass spectrum (m/z 70 eV) 332 (M+), determined, %,: C 86.92; H 4.75; N 8.33. C24H16N2. Calculated, %: C 86.71; H 4.86; N 8.43.
4’-(1-Naphthyl)2,3’-biquinolyl (5 b, C28H18N2)
As 4’-phenyl-2,3’-biquinolyl (5 a), from 0.48 g (1.25 mmol) 4’-(1-naphthyl)-1’,4’-dihydro-2,3’-biquinolyl (4 b) and 0.33 g (1.3 mmol) I2. Yield 0.44 g (92%), m.p. 158-159 °C (benzene-hexane). 1H NMR spectrum (CDCl3): 6.83 (1H, d, J34 = 8.55 Hz, 3-H); 7.31 (3H, m, 2’’-H, 3’’-H, 6’’-H); 7.39 (1H, d, J34 = 8.55 Hz, 4-H); 7.43-7.48 (3H, m, 7’’-H, 8’’-H, 6’-H); 7.63 (2H, d, J = 8.04 Hz, 4’’-H, 5’'-H); 7.68 (1H, d. d., J56 = 8.04, J67 = 7.03 Hz, 6-H); 7.71 (1H, d. d., J6'7' = 6.95, J7'8' =8.34 Hz, 7'-H); 7.73 (1H, d. d., J67=7.03, J78 =8.28 Hz, 7-H); 7.89 (1H, d, J56 = 8.04 Hz, 5-H); 7.92 (1H, d, J5'6' = 8.11 Hz, 5'-H); 8.10 (1H, d, J7'8' = 8.34 Hz, 8'-H); 8.28 (1H, d, J78 =8.28 Hz, 8-H); 9.55 (1H, s, 2'-H); mass spectrum (m/z 70 eV) 382 (M+), determined, %,: C 88.17; H 4.53; N 7.3. C28H18N2. Calculated, %: C 87.92; H 4.75; N 7.33.
4’-(2-Pyridyl)-2,3’-biquinolyl (5 c, C23H15N3)
As 4’-phenyl-2,3’-biquinolyl (5 a), from 0.42 g (1.25 mmol) 4’-(2-pyridyl)-1’,4’-dihydro-2,3’-biquinolyl (4 c) and 0.33 g (1.3 mmol) I2. Yield 0.4 g (94%), m.p. 93-94 °C (bnz). 1H NMR spectrum (CDCl3): 7.08 (1H, d, J34 = 8.54 Hz, 3-H); 7.18 (1H, d. d., J3’’4’’ = 7.69, J3’’5’' = 1.28 Hz, 3’’-H); 7.31 (1H, d. t., J4’’5’’ = 7.54, J5’’6’’ = 4.7, J3’’5’' = 1.28 Hz, 5’’-H); 7.55 (1H, d. d., J56 = 8.04, J67 = 7.01 Hz, 6-H); 7.59 (1H, d. t., J3’’4’’ = 7.69, J4’’5’’ =7.54, J4’’6’' = 1.7 Hz, 4’’-H); 7.71 (1H, d. d., J6'7' = 6.95, J7'8' =8.34 Hz, 7'-H); 7.73 (1H, d. d., J5'6' =8.11, J6'7'= 6.95 Hz, 6'-H); 7.74 (1H, d. d., J67=7.01, J78 =8.32 Hz, 7-H); 7.76 (1H, d, J56 = 8.04 Hz, 5-H); 7.79 (1H, d, J5'6' = 8.11 Hz, 5'-H); 7.91 (1H, d, J34 = 8.54 Hz, 4-H); 8.11 (1H, d, J7'8' = 8.34 Hz, 8'-H); 8.26 (1H, d, J78 =8.32 Hz, 8-H); 8.81 (1H, d. d., J5’’6’’ = 4.7, J4’’6’’ = 1.7 Hz, 6’’-H); 9.43 (1H, s, 2'-H); mass spectrum (m/z 70 eV) 333 (M+), determined, %,: C 82.89; H 4.47; N 12.64. C23H15N3. Calculated, %: C 82.85; H 4.54; N 12.61.
4’-(1-Methyl-2-benzimidazolyl)-2,3’-biquinolyl (5 d, C26H18N4)
As 4’-phenyl-2,3’-biquinolyl (5 a), from 0.48 g (1.25 mmol) 4’-(1-methyl-2-benzimidazolyl)-1’,4’-dihydro-2,3’-biquinolyl (4 d), and 0.33 g (1.3 mmol) I2. Yield 0.46 g (95%), m.p. 338-340 °C (EtOH). 1H NMR spectrum (CDCl3): 3.25 (1H, s, Me); 7.31 (1H, d, J5'6' = 8.1 Hz, 5'-H); 7.32 (1H, d, J34 = 8.4 Hz, 3-H); 7.38 (1H, d. t., J4’’5’’ = 8.52, J5’’6’’ = 7.41, J5’’7’’ = 1.61 Hz, 5’’-H); 7.51 (1H, d. d., J5'6' =8.1, J6'7'= 6.94 Hz, 6'-H); 7.53 (1H, d. d., J6’’7’' =7.65, J5’’7’' = 1.61 Hz, 7’'-H); 7.55 (1H, d. d., J56 = 8.06, J67 = 7.14 Hz, 6-H); 7.58 (1H, d. t., J5’’6’’ = 7.41, J6’’7’’ = 7.61, J4’’6’’ = 2.23 Hz, 6’’-H); 7.68 (1H, d. d., J6'7' = 6.94, J7'8' =8.32 Hz, 7'-H); 7.73 (1H, d, J56 = 8.06 Hz, 5-H); 7.82 (1H, d. d., J67=7.14, J78 =8.21 Hz, 7-H); 7.87 (1H, d. d., J4’'5’' =8.52, J4’'6’' = 2.23 Hz, 4’'-H); 7.93 (1H, d, J7'8' = 8.32 Hz, 8'-H); 7.94 (1H, d, J34 = 8.4 Hz, 4-H); 8.29 (1H, d, J78 =8.21 Hz, 8-H); 9.61 (1H, s, 2'-H); mass spectrum (m/z 70 eV) 386 (M+), determined, %,: C 80.94; H 4.59; N 14.47. C26H18N4. Calculated, %: C 80.81; H 4.69; N 14.5.
4’-(1-iso-Propyl-2-benzimidazolyl)-2,3’-biquinolyl (5 e, C28H22N4)
As 4’-phenyl-2,3’-biquinolyl (5 a), from 0.52 g (1.25 mmol) 4’-(1-iso-propyl-2-benzimidazolyl)-1’,4’-dihydro-2,3’-biquinolyl (4 e) and 0.33 g (1.3 mmol) I2. Yield 0.47 g (91%), m.p. 221-222 °C (bnz). 1H NMR spectrum (CDCl3): 0.72 (3H, d, J= 6.83, Me); 1.24 (3H, d, J= 6.83, Me); 4.16 (1H, m, CH); 7.31 (1H, d, J5'6' = 8.14 Hz, 5'-H); 7.36 (1H, d. t., J4’’5’’ = 8.55, J5’’6’’ = 7.38, J5’’7’’ = 1.63 Hz, 5’’-H); 7.43 (1H, d, J34 = 8.78 Hz, 3-H); 7.5 (1H, d. d., J6’’7’' =7.67, J5’’7’' = 1.63 Hz, 7’'-H); 7.52 (1H, d. d., J5'6' =8.14, J6'7'= 6.97 Hz, 6'-H); 7.55 (1H, d. d., J56 = 8.04, J67 = 7.11 Hz, 6-H); 7.59 (1H, d. t., J5’’6’’ = 7.38, J6’’7’’ = 7.67, J4’’6’’ = 2.25 Hz, 6’’-H); 7.7 (1H, d. d., J6'7' = 6.97, J7'8' =8.31 Hz, 7'-H); 7.74 (1H, d, J56 = 8.04 Hz, 5-H); 7.81 (1H, d. d., J67=7.11, J78 =8.21 Hz, 7-H); 7.92 (1H, d. d., J4’'5’' =8.55, J4’'6’' = 2.25 Hz, 4’'-H); 7.93 (1H, d, J34 = 8.78 Hz, 4-H); 8.06 (1H, d, J7'8' = 8.31 Hz, 8'-H); 8.28 (1H, d, J78 =8.21 Hz, 8-H); 9.57 (1H, s, 2'-H); mass spectrum (m/z 70 eV) 414 (M+), determined, %,: C 81.24; H 5.27; N 13.49. C28H22N4. Calculated, %: C 81.13; H 5.35; N 13.52.