Stereochemistry is about the shape and change of shape of molecules. There are several textbooks on this topic. This recent book has a unique feature: Several months after its publication (It was pub-lished in October 1998), the author has already established a website [
1] for this book at
http://www.bangor.ac.uk/ch/mnhome.htm where enhanced diagrams, answers to problems and an up-dated bibliography are provided and are freely accessible.
This book can be used as reading material for students taking organic chemistry courses and for graduate students. Organic chemists like me can also brush up their knowledge on stereochemistry, particularly organic stereochemistry. The copy I received is a paper-back version. The size and the volume of this book also make reading it comfortable. At the end of each chapter, references for fur-ther reading are provided, including a reference to the appropriate chapter of the definative stereo-chemistry text
Stereochemistry of Organic Compounds by Eliel and Wilen [
2]
The textbook currently retails at £ 25.00 and can be ordered directly from the publisher by calling +44 1242 267283, faxing +44 1242 253695. You can also order by e-mailing (
[email protected]).
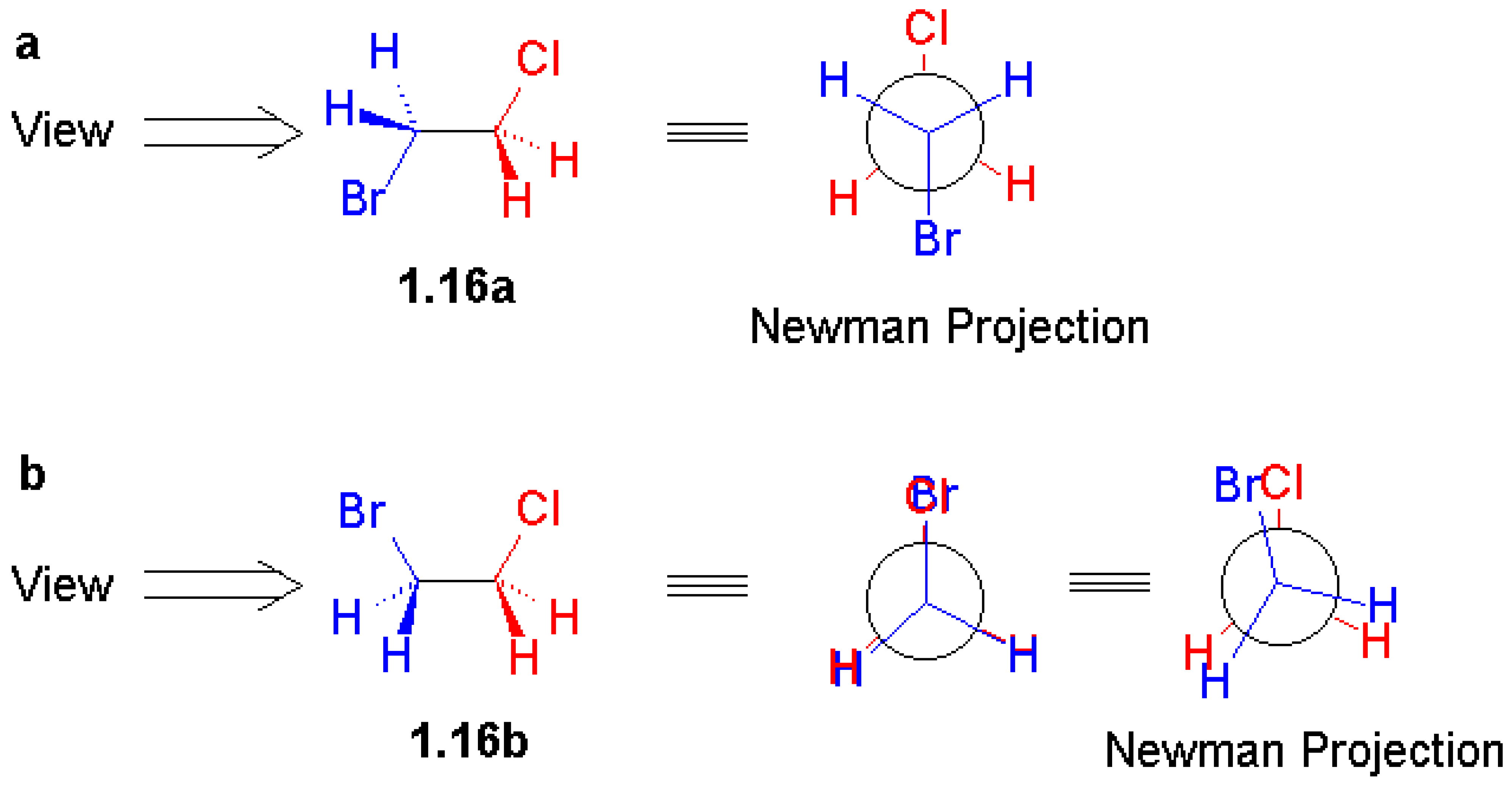
I am always interested in how the authors of stereochemistry books use stereochemical representa-tions, because the solid wedge, broken wedge, broken line, solid bar, and broken bar are all frequently used for structural drawing. A large number of combinations of these representations have been seen in literature, which have caused confusion and ambiguity [
3]. In this text, stereochemical representa-tion is introduced in Chapter 1. The first figure defines correct (black and gray) and incorrect (red or blue) representations of a tetrahedron (also at the
http://www.bangor.ac.uk/ch/mn/booknet/chapter1/c1fig.htm website).
Figure 1.4 of the book, which is reproduced here as
Figure 2, is a representative and nice illustra-tion of the graphics contained in this book. Unfortunately the print version has graphics in black and white. Therefore, you may wish to visit this book's website [
1], enjoying its colourful figures and 3D molecular structures and further enhanced graphics.
As mentioned before, there are obvious two aspects (structures and processes, or shape and shape change) to stereochemistry. Chapter 5 is mainly a discussion on stereochemical processes. Chemical analysis and separation of a mixture always depend on the differences in the properties of the compo-nents: the more
different (distinguishable) the components, the easier they are to separate and analyse. The spontaneity of the opposite process (here racemization, for instance) relies on the components similarity (or indistinguishability): the more
similar, more miscible. I have criticized the thermody-namic theory of these two types of process [
3]. The tacit belief that the spontaneity of a mixing proc-ess is driving by entropy due to the components' difference is highly disputable. Enantiomers are very much the same in almost all properties, except optical rotation. The chemical modification by dias-tereomerization will enhance differences and separate a racemic mixture. On each occaision that I read a nice text book on stereochemistry, I am impressed by the striking facts of stereochemical processes involving mixing and separation.
The complementarities of enantiomeric compounds with an enzyme or a receptor (original Fig. 3.8, ref.1, p.53) or with a chiral stationary phase (original Fig. 5.4, ref.1, p.107), and between pairs (origi-nal Fig. 8.13, p.186) are well presented. In Pauling's classical The Nature of the Chemical Processes, there are only two important keywords, one is resonance, the other is complementarity. The theoretical problems of both resonance (e.g., the rapid degenerate Cope rearrangement in the area of dynamic stereochemistry, which is not covered here due to this book's limited volume) and complementarity are still outstanding.
Finally, this book contributed almost three pages to the very interesting problem of the origin of biomolecular chirality (ref.1, p.54). Physicists call it a symmetry breaking phenomenon. It happened either by chance or not by chance. A recent work by Szabo-Nagy and Keszthelyi [
5], if confirmed, would be a great breakthrough in the understanding of the molecular asymmetry evolution of bio-sphere.