A New Pathway to 3-Hetaryl-2-oxo-2H-chromenes: On the Proposed Mechanisms for the Reaction of 3-Carbamoyl-2-iminochromenes with Dinucleophiles
Abstract
:Introduction
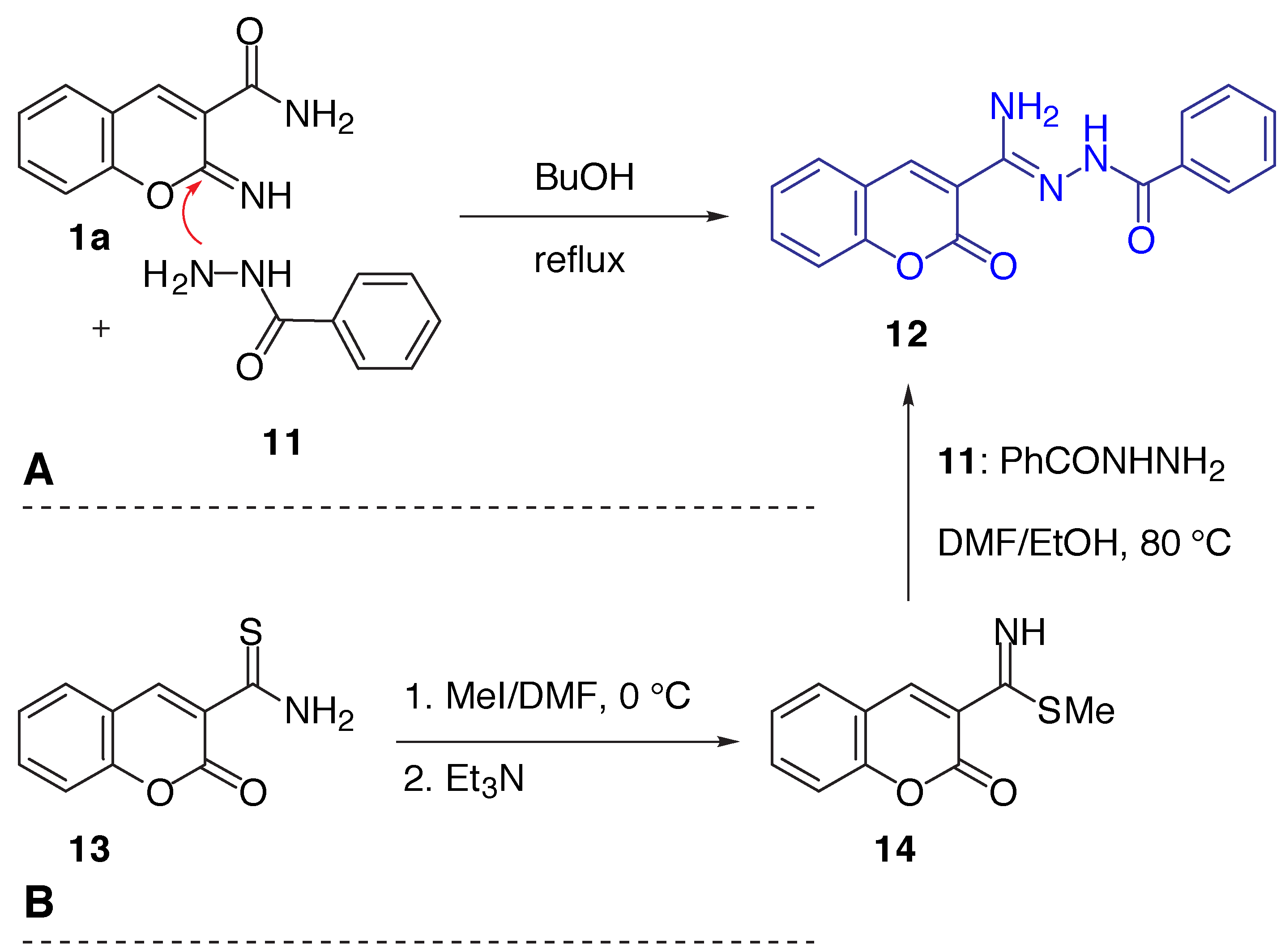
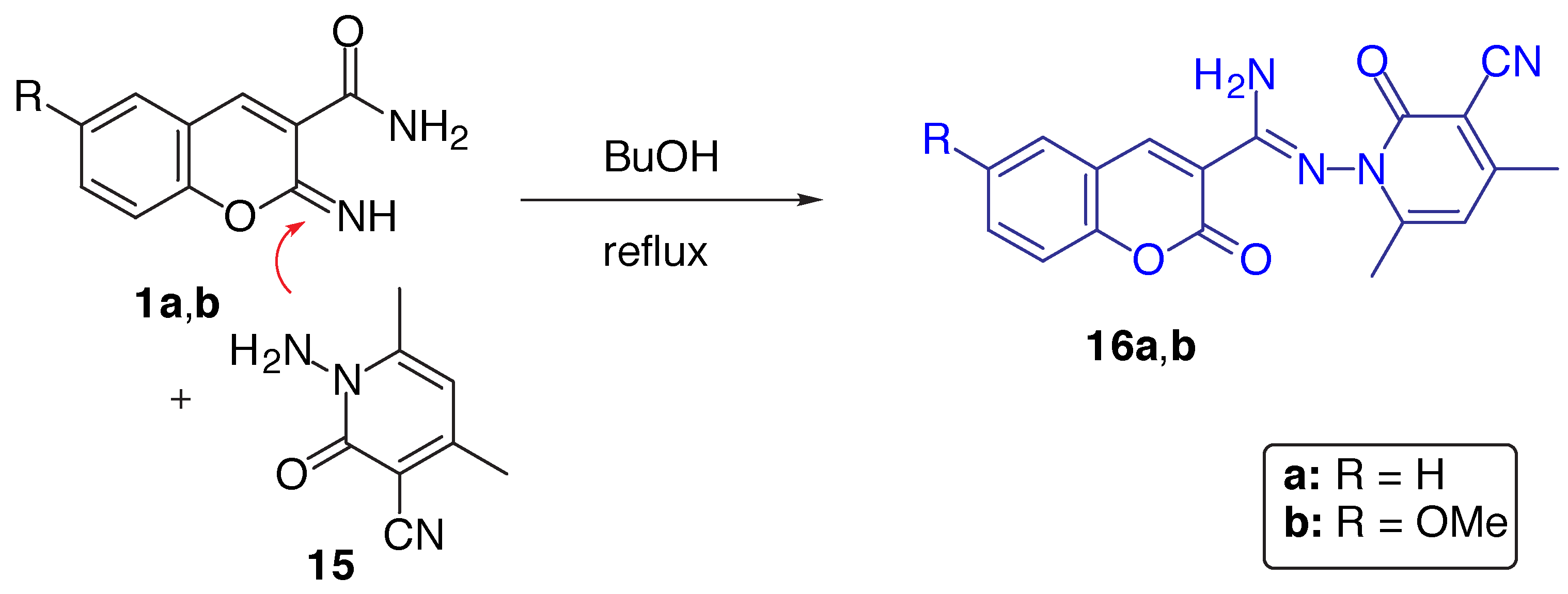
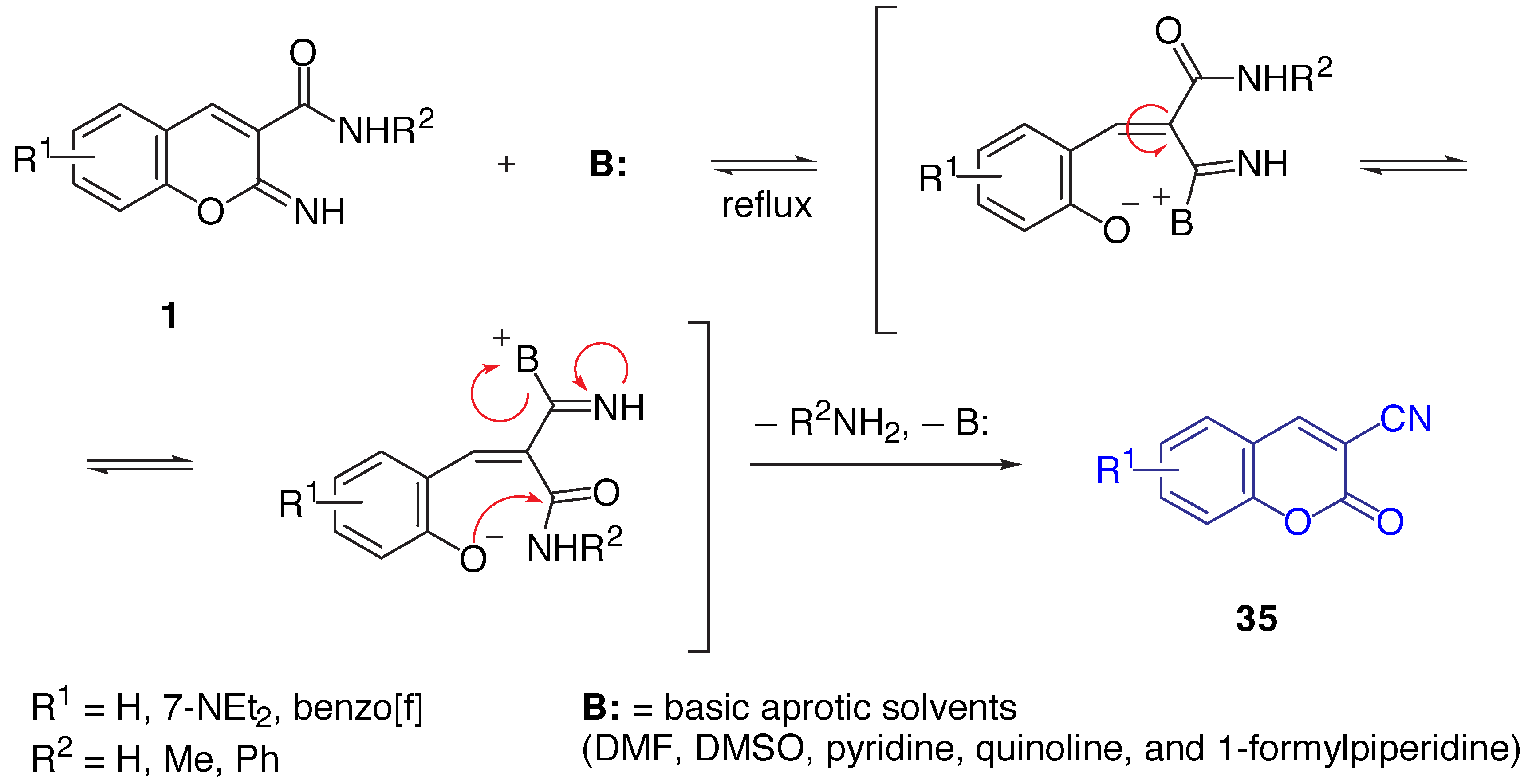
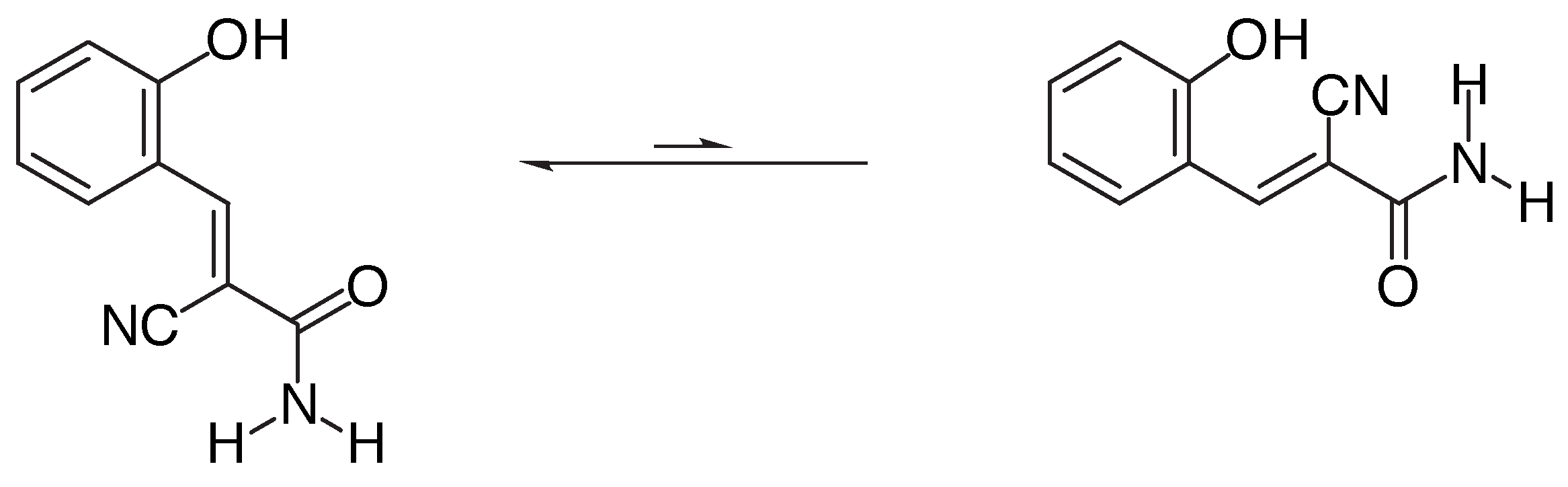
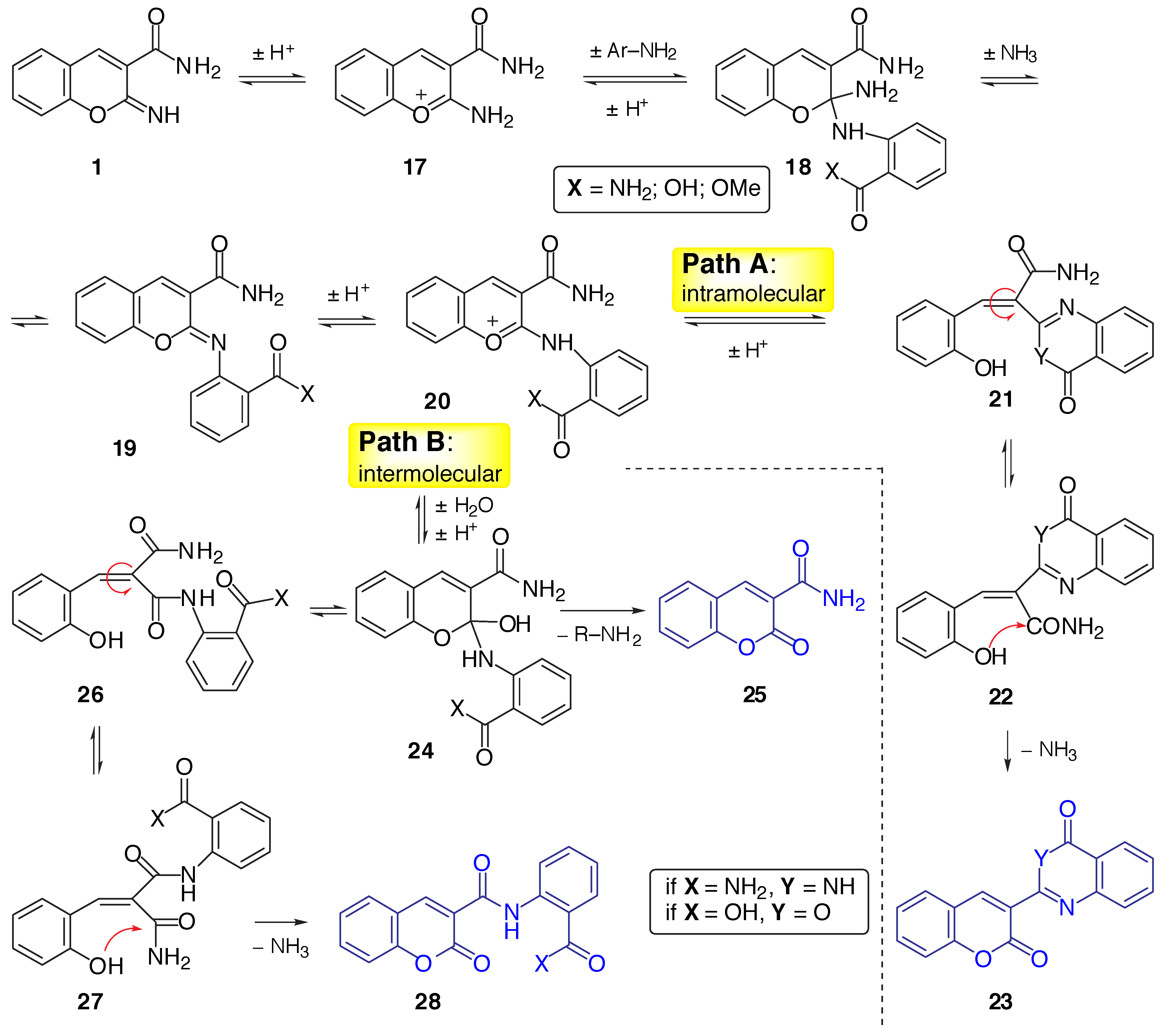
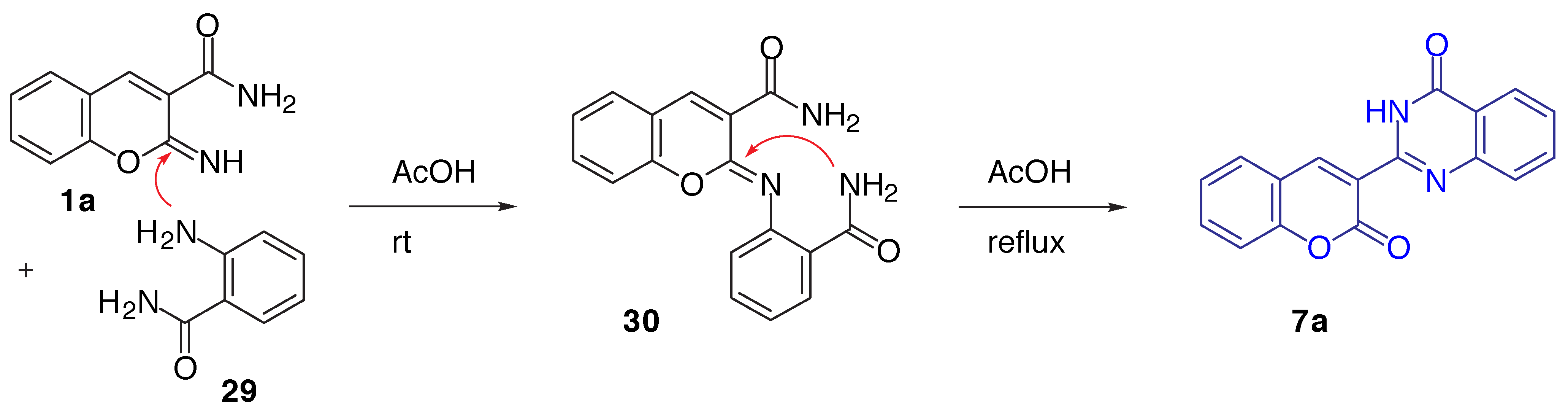
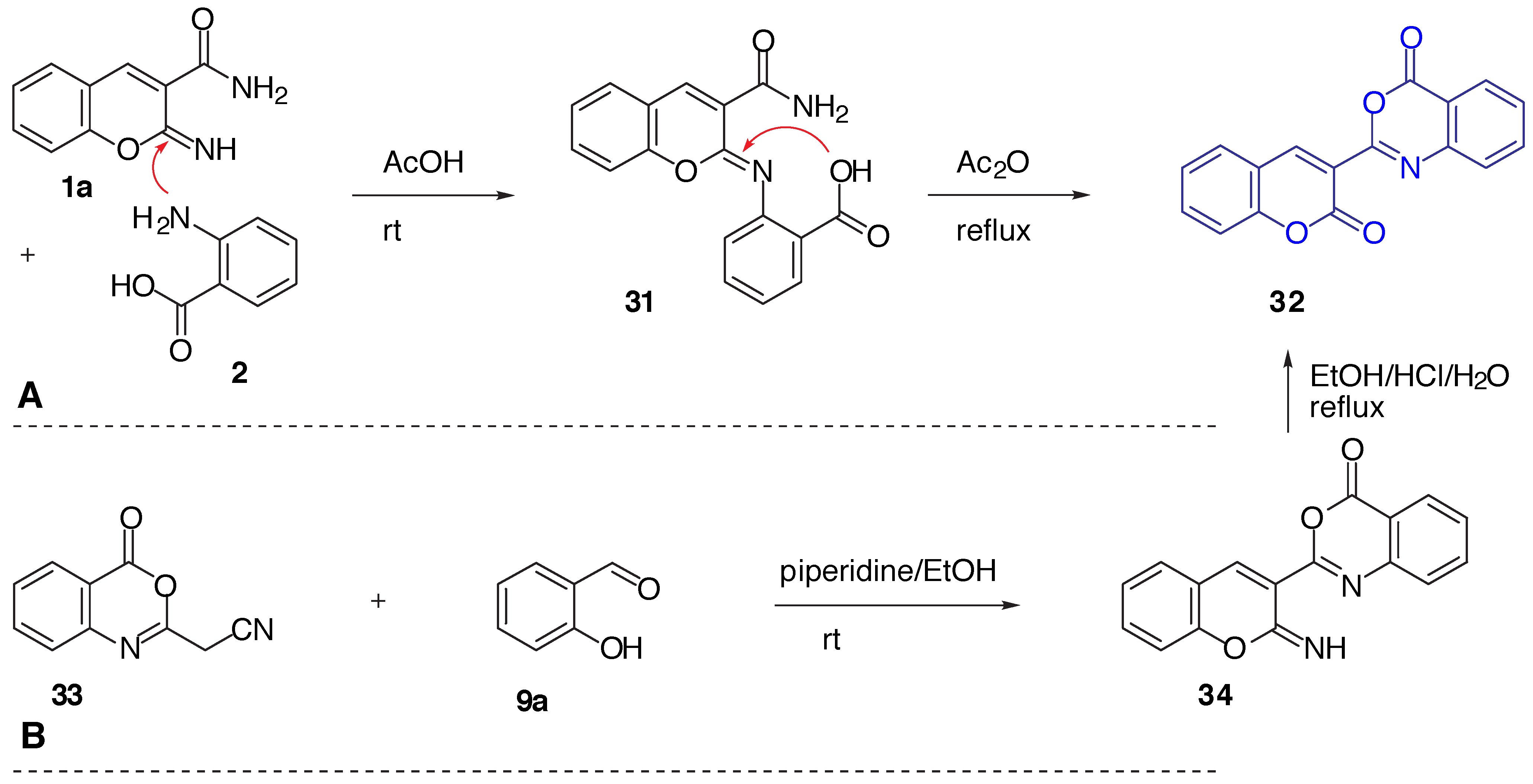
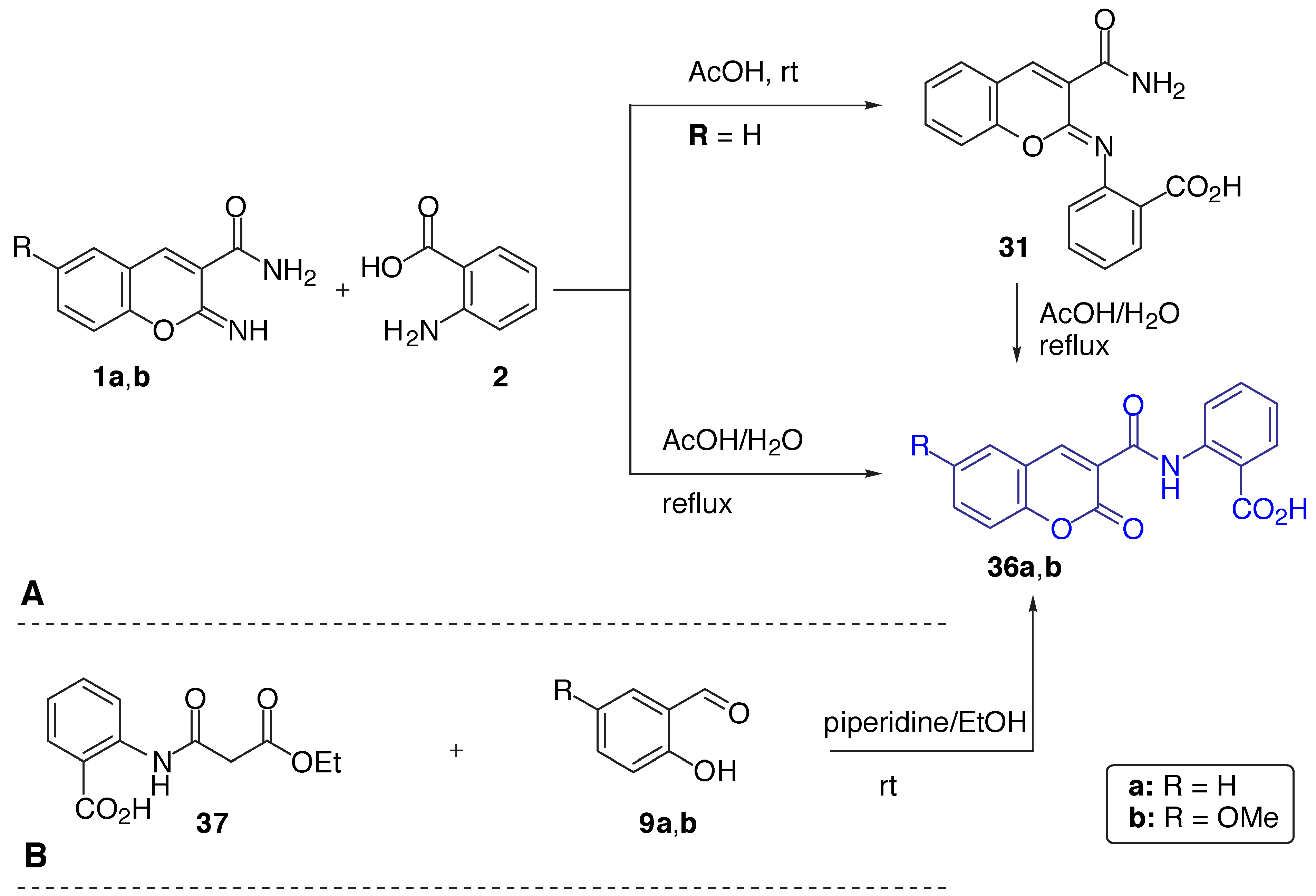
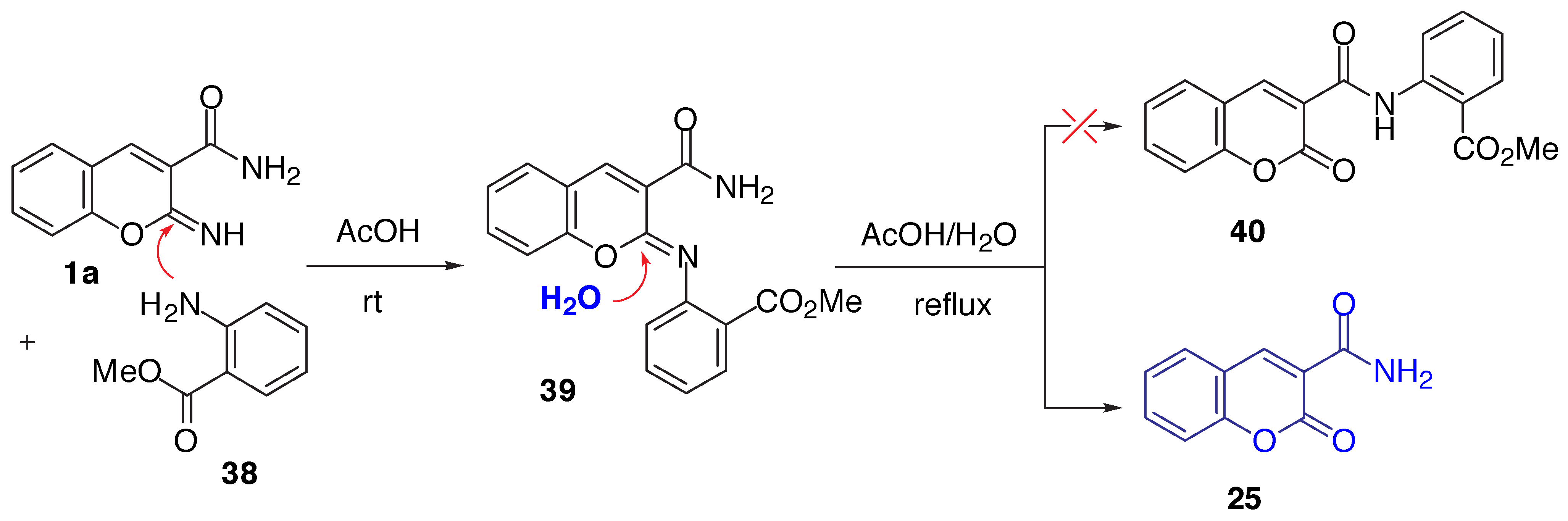
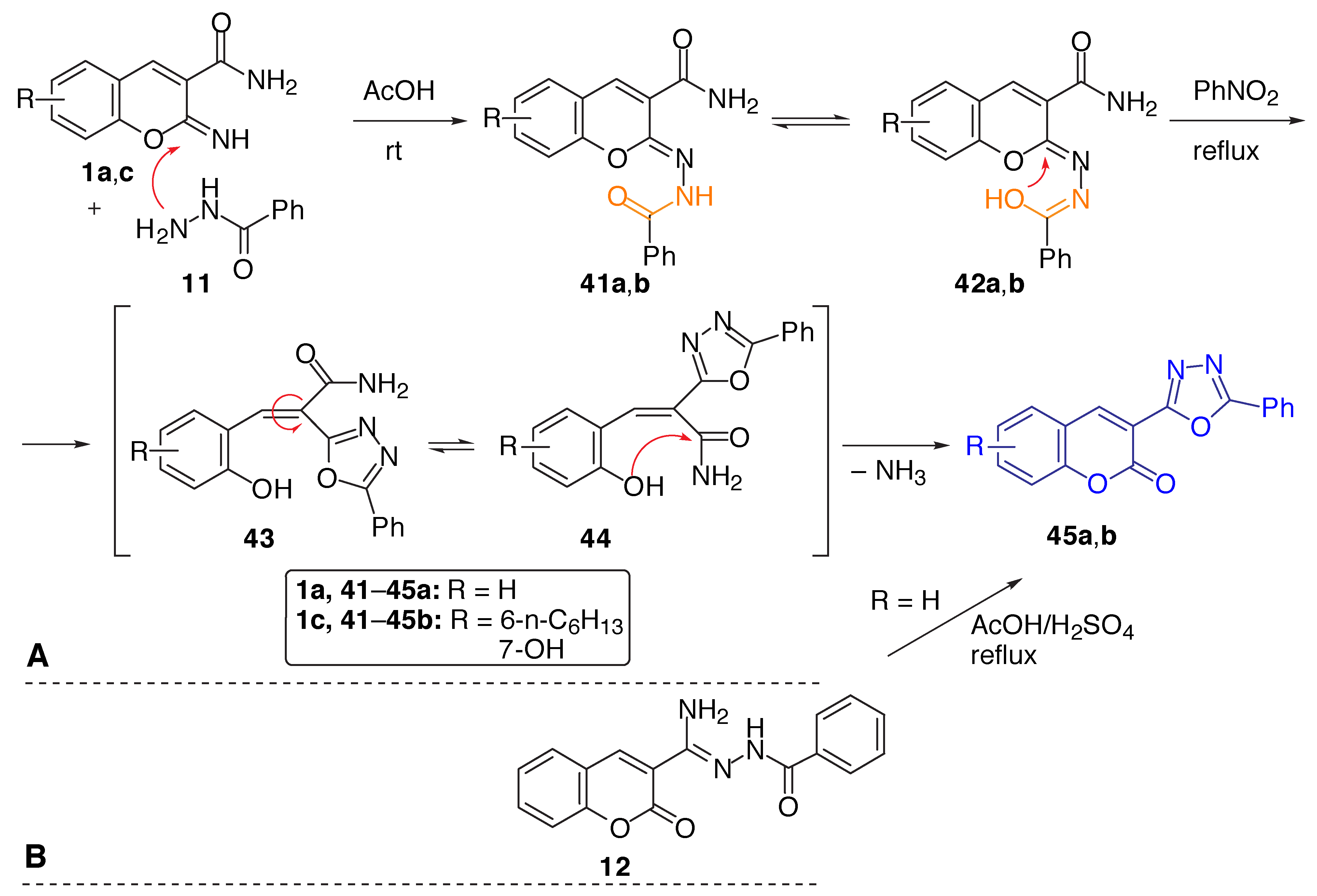
Results and Discussion

Conclusion
Experimental
General
General procedure
General procedures
References and Notes
- Recent reviews on naturally occurred coumarins: Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins. Plants, structure, properties. Khim. Prir. Soedin. 1998, 560–593. [Google Scholar] Malikov, V.M.; Saidkhodzhaev, A.I. Physical constants and spectral data of coumarins. Part 2. Coumarins: plants, structure, properties. Khim. Prir. Soedin. 1998, 384–432. [Google Scholar] Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins: plants, structure, properties. Part 1. Coumarin distribution in plants. Khim. Prir. Soedin. 1998, 250–281, [Engl. Transl.: Chem. Nat. Compd. 1998, 34, 202-264. [Google Scholar] Estevez-Braun, A.; Gonzalez, A.G. Coumarins. Nat. Prod. Rep. 1997, 14, 465–475. [Google Scholar] [CrossRef] [PubMed]Murray, R.D.H. Naturally occurring plant coumarins. Prog. Chem. Org. Nat. Prod. 1997, 72, 1–119. [Google Scholar]
- O’Kennedy, R.; Thornes, R.D. Coumarins: biology, applications, and mode of action; John Wiley & Sons: Chichester; New York, 1997. [Google Scholar]
- Zubkov, V.A.; Kovalenko, S.N.; Chernykh, V.P.; Ivkov, S.M. New derivatives of coumarin: 2-(N-R-imino)-2H-1-benzopyrans. Khim. Geterotsikl. Soedin. 1994, 760–766, [Engl. Transl.: Chem. Heterocycl. Compd. (N. Y.) 1994, 30, 665-670]. [Google Scholar] [CrossRef]
- Kovalenko, S.N.; Zubkov, V.A.; Chernykh, V.P.; Turov, A.V.; Ivkov, S.M. Recyclization of 2-imino-2H-1-benzopyrans under the influence of nucleophilic reagents. 1. New approach to the synthesis of 3-(1,3,4-oxadi-, thiadi-, and triazolyl-2)coumarins. Khim. Geterotsikl. Soedin. 1996, 186–192, [Engl. Transl.: Chem. Heterocycl. Compd. (N. Y.) 1996, 32, 163-168]. [Google Scholar] Kovalenko, S.N.; Chernykh, V.P.; Shkarlat, A.E.; Ukrainets, I.V.; Gridasov, V.I.; Rudnev, S. Recyclization of 2-imino-2H-1-benzopyrans under the influence of nucleophilic reagents. 2. Reaction of 2-iminocoumarin-3-carboxamides with o-aminobenzenesulfonamide. Khim. Geterotsikl. Soedin. 1998, 916–920, [Engl. Transl.: Chem. Heterocycl. Compd. (N. Y.) 1998, 34, 791-795]. [Google Scholar] [CrossRef] Bilokin, Y.V.; Kovalenko, S.N.; Bylov, I.E.; Chernykh, V.P. Rearrangements of 2-imino-2H-1-benzopyran-3-carboxamides under action of anthranilic acid as N-nucleophile. Heterocycl. Commun. 1998, 4, 257–260. [Google Scholar] [CrossRef] Kovalenko, S.N.; Vasil’ev, M.V.; Sorokina, I.V.; Chernykh, V.P.; Turov, A.V.; Rudnev, S.A. Recyclization of 2-imino-2H-1-benzopyrans using nucleophilic reagents. 3. Reaction of 2-iminocoumarin-3-carboxamides with o-phenylenediamines and o-amino(thio)phenols. Khim. Geterotsikl. Soedin. 1998, 1664–1667, [Engl. Transl.: Chem. Heterocycl. Compd. (N. Y.) 1998, 34, 1412-1415]. [Google Scholar] [CrossRef] Kovalenko, S.N.; Sytnik, K.M.; Nikitchenko, V.M.; Rusanova, S.V.; Chernykh, V.P.; Porokhnyak, A.O. Recyclization of 2-imino-2H-1-benzopyrans by the action of nucleophilic reagents. 4. Use of 2-(N-aroylhydrazono) coumarin-3-carboxamides for the synthesis of 3-(1,3,4-oxadiazol-2-yl)coumarins. Khim. Geterotsikl. Soedin. 1999, 190–193, [Engl. Transl.: Chem. Heterocycl. Compd. (N. Y.) 1999, 35, 167-170]. [Google Scholar] [CrossRef] Vasylyev, M.V.; Bilokin, Y.V.; Branytska, O.V.; Kovalenko, S.M.; Chernykh, V.P. A facile method for synthesis of heterocycles containing tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine and coumarin moieties. Heterocycl. Commun. 1999, 5, 241–242. [Google Scholar] [CrossRef] Bilokin, Y.V.; Vasylyev, M.V.; Branytska, O.V.; Kovalenko, S.M.; Chernykh, V.P. A novel and expedient approach to new heterocycles containing benzothiophene, benzothieno[2,3-d]pyrimidine and coumarin moieties. Tetrahedron 1999, 55, 13757–13766. [Google Scholar] [CrossRef] Sytnik, K.M.; Kovalenko, S.M.; Chernykh, V.P.; Goncharova, V.M. Synthesis and tuberculostatic activities of 2-(R-thiosemicarbazono)coumarin-3-carboxamides. Fiziologichno Aktyvni Rechovyny 1999, (2), 50–53, (in Ukrainian with Engl abstr.). [Google Scholar] Kovalenko, S.N.; Bylov, I.E.; Belokon, Ya.V.; Chernykh, V.P. Recyclization of 2-imino-2H-1-benzopyrans under the action of nucleophilic reagents. 5. Interaction of 2-imino-2H-1-benzopyran-3-carboxamide with anthranilic acid and its derivatives. Khim. Geterotsikl. Soedin. 2000, 1175–1181. [Google Scholar]
- Selim, M.R.; Aly, F.M.; Bedair, A.H.; Abu-Shanab, F.A. Synthesis of some benzoxazin-4-one derivatives and study of their reaction with nucleophilic reagents. J. Indian Chem. Soc. 1992, 69, 688–690. [Google Scholar] [CrossRef]
- El-Hashash, M.A.; Kaddah, A.M.; El-Kady, M.; Ammer, M.M. Some reactions of 3[2′-(4′H,2′,1′)-benzoxazin-4′-only]coumarins and 3(2′-quinazol-4′-onyl)coumarins. Pak. J. Sci. Ind. Res. 1982, 25, 104–108. [Google Scholar]
- Enomoto, S.; Sato, K.; Suzuki, G. 3-(4-Oxo-3,4-dihydro-2-quinazolinyl)-7-diethylaminocoumarin dyes. Ger. Offen. 2,005,933 (1970); Chem. Abstr. 1971, 74, 65585. [Google Scholar]
- Kametani, T.; Loc, C.V.; Higa, T.; Koizumi, M.; Ihara, M.; Fukumoto, K. Iminoketene cycloaddition. 2. Total synthesis of arborine, glycosminine, and rutecarpine by condensation of iminoketene with amides. J. Am. Chem. Soc. 1977, 99, 2306–2309. [Google Scholar] [CrossRef]
- Schiemenz, G.P. Zur Reaktion von 2-Hydroxy-benzaldehyd mit Cyanacetamid und Malodinitril. Chem. Ber. 1962, 95, 483–486. [Google Scholar] [CrossRef]
- Abdel Aziz, M.A.; Daboun, H.A.; Abdel Gawad, S.M. α-Cyanothioacetamide and its derivatives in heterocyclic synthesis: preparation of several new 4-oxoquinazoline derivatives. J. Prakt. Chem. 1990, 332, 610–618. [Google Scholar] [CrossRef]
- A similar process on formation of 3-amino-2-cyano-(2-imino-4-methyl-2H-1-benzopyran-3-yl)prop-2-enenitrile by a reaction of 2-imino-4-methyl-2H-1-benzopyran-3-carbonitrile with malononitrile, acting as C-nucleophile, was reported: O’Callaghan, C.N.; McMurry, T.B.H.; O’Brien, J.E.; Draper, S.M.; Wilcock, D.J. Formation of polyheterocyclic systems by reaction of 2-imino-4-methyl-2H-1-benzopyran-3-carbonitrile with active methylene compounds. J. Chem. Soc., Perkin Trans. 1 1996, 1067–1071. [Google Scholar]
- For a leading list of references on preparation of coumarins and other fused α-pyrones from 3-(2-hydroxyaryl)propenoic esters through their thermal E/Z isomerization, see: Cartwright, G.A.; McNab, H. Synthesis of coumarins by flash vacuum pyrolysis of 3-(2-hydroxyaryl)propenoic esters. J. Chem. Res. (S) 1997, 296–297. [Google Scholar]
- Brunskill, J.S.A.; De, A.; Elagbar, Z.; Jeffrey, H.; Ewing, D.F. Convenient synthesis of coumarin-3-thiocarboxamides. Synth. Commun. 1978, 8, 533–539. [Google Scholar] [CrossRef]
- Silin, A.V.; Gorobets, N.Y.; Sytnik, K.M.; Nikitchenko, V.M. Recyclization of substituted 2-iminocoumarin-3-carboxamides in aprotic basic solvents. Visn. Khark. Univ. 1997, 395, 274–277, Chem. Abstr. 1998, 129, 290037. [Google Scholar]
- O’Callaghan, C.N.; McMurry, T.B.H.; O’Brien, J.E. Isomerisation of benzopyran-2-imines in [2H6]dimethyl sulfoxide. J. Chem. Soc., Perkin Trans. 2 1998, 425–429. [Google Scholar] [CrossRef]
- Hand, E.S.; Jencks, W.P. Mechanism of the reaction of imido esters with amines. J. Am. Chem. Soc. 1962, 84, 3505–3514. [Google Scholar] [CrossRef]
- Kuhn, R.; Weiser, D. Lactonacetale, eine neue Klasse von Carbonsäure-Derivaten. Angew. Chem. 1957, 69, 371–376. [Google Scholar] [CrossRef]
- Karasev, A.A.; Lukatskaya, L.L.; Rubtsov, M.I.; Zhikol, E.K.; Yarmolenko, S.N.; Ponomarev, O.A. Synthesis, protolytic equilibrium and stability of 2-amino-3-(2-benzimidazolyl)-1-benzopyrylium salts in aqueous alcoholic medium. Zh. Obshch. Khim. 1995, 65, 1547–1557, Chem. Abstr. 1996, 124, 342517. [Google Scholar] Rubtsov, M.I.; Lukatskaya, L.L.; Karasev, A. Structure and reactivity of 2-amino-3-carbamoylchromenylium salts. Zh. Obshch. Khim. 1999, 69, 138–141, [Engl. Transl.: Russ. J. Gen. Chem. 1999, 69, 134-137]. [Google Scholar]
- Matlack, A.S. Nickel salts of iminoheterocyclic amides. Ger., Offen. 1,806,435 (1969); Chem. Abstr. 1970, 72, P4337. [Google Scholar]
- O’Callaghan, C.N. Isomerization of 2-aryl-4-oxo-2,3-dihydrobenzopyrano[2,3-d]pyrimidines to 2-aryl-4-hydroxy-5-H-benzopyrano[2,3-d]pyrimidines. J. Chem. Soc., Perkin Trans. 1 1980, 1335–1337. [Google Scholar] [CrossRef]
- Review: Coppola, G.M. The chemistry of 4H-3,1-benzoxazin-4-ones. J. Heterocycl. Chem. 1999, 36, 563–588. [Google Scholar] [CrossRef] Abdalla, M.M.; Elkady, M.; El-Farargy, A.F. Synthesis of some 3-(3′,1′-benzoxazin-4′-one)-6-substituted coumarins and their chemical reactions. Egypt. J. Chem. 1977, 20, 245–257. [Google Scholar] [CrossRef]
- Ossman, A.E.; El-Zahabi, M.M.; El-Hakim, A.E.; Osman, A.N. Synthesis of 2-cyanomethyl-3,1-benzoxazin-4(H)-one. Egypt. J. Chem. 1988, 31, 381–385. [Google Scholar]
- Ukrainets, I.V.; Bezugly, P.A.; Treskach, V.I.; Taran, S.G.; Gorokhova, O.V. Ethyl-esters of malonanilic acids – synthesis and pyrolysis. Tetrahedron 1994, 50, 10331–10338. [Google Scholar] [CrossRef]
- Review: Fry, D.W. Recent advances in tyrosine kinase inhibitors. Annu. Rep. Med. Chem. 1996, 31, 151–160. [Google Scholar]
- Czerney, P.; Hartmann, H. Zur Darstellung von 3-Cyancumarinen. J. Prakt. Chem. 1981, 323, 691–693. [Google Scholar] [CrossRef]
- O’Callaghan, C.N.; Conalty, M.L. Anticancer agents. XIII. Synthesis and antitumor activity of 2-iminochromene derivatives. Proc. R. Ir. Acad., Sect. B 1979, 79B, 87–98. [Google Scholar]
- Reid, W.; Meyer, A. Über die Verwendung von Cyanacethydrazid zur Darstellung von Stickstoffheterocyclen. I. Eine einfache Synthese von N-amino-α-pyridonen. Chem. Ber. 1957, 90, 2841–2848. [Google Scholar] [CrossRef]
- Nielsen, S.F.; Christensen, S.B.; Cruciani, G.; Kharazmi, A.; Liljefors, T. Antileishmanial chalcones: statistical design, synthesis, and three-dimensional quantitative structure-activity relationship analysis. J. Med. Chem. 1998, 41, 4819–4832. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.D.; Armarego, W.L.F. Purification of laboratory chemicals, 3d Eds ed; Pergamon Press: Oxford, 1988. [Google Scholar]
- El-Ahmad, Y.; Brion, J.-D.; Reynaud, P. Synthesis of N-(2-dialkylaminoethyl)-2-oxo-2H-1-benzo(thio)pyran-3-carboxamidines. Heterocycles 1993, 36, 1979–1988. [Google Scholar]
- Milcent, R. 1,3,4-Oxadiazoles. Synthesis of some polyheterocyclic compounds from (5-phenyl-1,3,4-oxadiazol-2-yl)acetic acid. Ann. Chim. (Paris) 1967, 2, 221–226, Chem. Abstr. 1968, 68, 49490. [Google Scholar]
- Samples Availability: Available from the authors.
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kovalenko, S.M.; Bylov, I.E.; Sytnik, K.M.; Chernykh, V.P.; Bilokin, Y.V. A New Pathway to 3-Hetaryl-2-oxo-2H-chromenes: On the Proposed Mechanisms for the Reaction of 3-Carbamoyl-2-iminochromenes with Dinucleophiles. Molecules 2000, 5, 1146-1165. https://doi.org/10.3390/51001146
Kovalenko SM, Bylov IE, Sytnik KM, Chernykh VP, Bilokin YV. A New Pathway to 3-Hetaryl-2-oxo-2H-chromenes: On the Proposed Mechanisms for the Reaction of 3-Carbamoyl-2-iminochromenes with Dinucleophiles. Molecules. 2000; 5(10):1146-1165. https://doi.org/10.3390/51001146
Chicago/Turabian StyleKovalenko, Sergiy M., Igor E. Bylov, Konstantyn M. Sytnik, Valentyn P. Chernykh, and Yaroslav V. Bilokin. 2000. "A New Pathway to 3-Hetaryl-2-oxo-2H-chromenes: On the Proposed Mechanisms for the Reaction of 3-Carbamoyl-2-iminochromenes with Dinucleophiles" Molecules 5, no. 10: 1146-1165. https://doi.org/10.3390/51001146



