The Fourth School for Young Scientists on Chemistry of Porphyrins and Related Compounds Ivanovo 2000 and the Symposium “Application of Porphyrins in Medicine” were held at the facilities of Ivanovo State University of Chemistry and Technology and the Institute of the Solution Chemistry of the Russian Academy of Sciences in Ples (Ivanovo region, Russia) from August 21 to August 27, 2000. About a hundred lecturers and young scientists from Russia, Belarus, Ukraine, Italy and Poland took part in the various sessions of the School and Symposium. The main centers (Moscow, Ivanovo, St-Petersburg, etc.) and directions of porphyrin investigation were well represented by Russian lecturers. The School program included lectures on synthesis, physico-chemical, coordination and application properties of porphyrins, phthalocyanines and related macroheterocyclic compounds.
Lectures on synthetic chemistry of porphyrins and their analogs were given by Andrei F. Mironov: “Chlorins and porphyrins with additional six-membered exocycles”, Helij V. Ponomarev: “Novel surprising transformations of meso-substituted porphyrins and chlorins”, Zinaida I. Zhilina: “Synthesis, structure and application of water-soluble porphyrins”, Olga G. Khelevina: “Substitution reactions of tetrazaporphyrins”, Oskar I. Koifman: “Polymer-bound porphyrins” and Michail K. Islyaikin: “Synthesis and properties of substituted macroheterocyclic compounds”. Syntheses of porphyrin coordination compounds were reviewed by Alexander S. Semeykin in his lecture “Synthesis of metalloporphyrins and their analogs”, by Tatjana N. Lomova in “Redox reactions accompanying the complex formation reactions of porphyrins with metals” and Claudio Ercolani in “μ-Dimer phthalocyanine complexes”. The lecture of Oleg A. Golubchikov: “Kinetics and mechanisms of coordination reactions of porphyrins” was devoted to a related theme. Boris D. Berezin considered general questions of chemistry of coordination compounds in his lecture “Modern state of chemistry of nontraditional classes of coordination compounds. Multi-nuclear complexes. Spherands.”
Different aspects of spectroscopy of porphyrins and their analogs were covered by Alexander A. Krasnovsky: “Mechanisms and application of delayed phthalocyanine fluorescence induced by singlet oxygen”, Edward I. Zenkevich: “Physico-chemical problems of primary events in photosynthesis”, Nikolai I. Kruk: “Cation-anion interactions of watersoluble porphyrins in solutions”, Nugzar Zh. Mamardashvili: “NMR spectra of porphyrins” and Svetlana A. Siling: “Heteroazocyclanes – bifluorophores and trifluorophores: Synthesis, photophysical properties, non-radiational transfer of energy of electron excitation in macrocycle and on lanthanides”. Aspects of the physical chemistry of porphyrins were discussed in the lectures of Vladimir G. Andrianov: “Acid-base properties of porphyrins” and Michail B. Berezin: “Principles of formation of solvate surrounding of complex organic molecules”.
Considerable attention was given at the School to catalysis and electrocatalysis by porphyrins. Anna B. Solovieva gave a lecture entitled “Catalytic systems on the base of immobilized porphyrins”, while Vera K. Skachkova - “Metalloporphyrins and its structural analogs as multifunctional modifiers for polyacrylonitrile thermostructurization” and Stefan Kurek - “Modification of Electrodes using Porphyrins”, also covered this area. The lectures of Galina I. Shumilova: “Metalloporphyrins in ionometry”, Alexander B. Valiotti: “Chemical sensors on the base of porphyrins and their analogs” and Michail I. Bazanov: “Electrochemical properties of macroheterocyclic compounds” were devoted to the electrochemistry of porphyrins and related compounds.
The Program of the Symposium “Application of Porphyrins in Medicine” included both lectures and posters. Rimma P. Evstigneeva gave a lecture on a theme “Carbonylporphyrins and perspectives of their use for boron-neutron therapy of cancer”, Dmitry B. Berezin – “Elaboration of the methods of immobilization of porphyrins and chlorins on polymer supports for blood sterilization”, Ludmila A. Grubina – “Influence of radiation on porphyrin metabolism in human organism”, Andrei F. Mironov – “Experience of clinic introduction of method of photodynamic therapy of cancer in Russia using “Photogem” preparation”
The Poster Session was held as joint feature of the School for Young Scientists and the Symposium. In total 36 posters devoted to the synthesis, properties and practical applications of porphyrins, pthtalocyanines and related compounds were presented.
The School-Symposium were organized with the financial support of the Federal Special Program “Integration” (grant N 262), the Russian Foundation for Fundamental Research (grant N 00-03-42037) and the sponsorship of the “Yakovlevskaja Manufactura” company, Privolzhsk (Ivanovo region, Russia).
Oleg A. Golubchikov and Tatjana A. Ageeva
Abstracts
Chlorins and Porphyrins with Six-Membered Exocyclic Rings
Andrei F. Mironov
M.V. Lomonosov Moscow State Academy of Fine Chemical Technology, Moscow, Russia
The introduction of additional exocyclic rings into natural porphyrins and chlorins, and as well their derivatives, results in substantial changes in the physical, chemical and biological properties of the initial compounds. Unlike natural pigments which retain their five and sevenmembered cyclic rings, the majority of chemical modifications are connected with the formation of six-membered cycles. In the present review basic attention will be given to similar chlorins and porphyrins. Rather symbolically they can be divided into compounds with cyclohexane, cyclohexene and cyclohexadiene rings, and also rings in which one or more Catoms are replaced by oxygen, nitrogen and/or other atoms. These rings can either be situated at the β-carbon atoms of the main macrocycle, or they can include meso-positions, that also influence sufficiently the properties of similar compounds.
Compounds with hexa-anhydride and imide cycles are of special interest among the considered chlorins. Attention will be given to their receptors, chemical transformations and properties. To conclude, the possibilities of using porphyrins and chlorins with additional exocycles as photosensitizers for photodynamic therapy of cancer will be examined.
Kinetics and Mechanisms of Coordination Reaction of Porphyrins
Oleg A. Golubchikov
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
Interaction of porphyrins with transition metal salts results ultimately in the formation of metalloporphyrins, which are very stable coordination compounds. Porphyrins have definite solubility in non-aqueous solutions and are insoluble in water because of high stability of their crystal lattices. Introduction of bulky substituents that loosen the crystal lattice can increase porphyrin solubility in non-aqueous media. Introduction of ionized groups into the molecular structure allows the one to obtain water-soluble compounds.
The metalloporphyrin formation process has specific features in aqueous and non-aqueous solutions. Because of large size and hydrophobic properties of porphyrin molecules in water they are inclined to form outer sphere complexes with hydrated metal cations. This reaction has a fast rate, which could be measured using special techniques for investigating kinetics of fast reactions. Corresponding experimental data are not numerous. In the outer sphere complexes structure H2P…[M(H2O)n]z+ due to influence of the electrostatic field of hydrated metal cation the porphyrin molecules undergo polarization, their reactivity increases and the rate of metalloporphyrin formation increases sharply.
In non-aqueous solutions formation of outer sphere complex compounds of porphyrins with metal salts is not typical. There are different types of salt – porphyrin interaction in these conditions. In chloroform, acetonitrile and similar solvents the transition metal salts could undergo hydrolysis under the action of traces of water. This leads to considerable increases of the medium acidity, porphyrin protonation and formation of specific interaction products. Depending on the ration of porphyrin and salt concentrations, porphyrin…salt complexes with 1:1, 1:2 or 2:1 composition could be formed, e.g. H3P+…[MX2OH(Solv)3]-, H4P2+…{[MX2OH(Solv)3]-}2 or {H3P+}2…[MX2OH(Solv)2]2-. Protonated porphyrin forms are kinetically inert and could not transfer to metalloporphyrins.
When no salt hydrolysis or solvolysis processes take place, the structure and stability of the coordination sphere of the metal solvatocomplex become the main factors determining the rate of metalloporphyrin formation in non-aqueous solutions. This question is given special attention in the review.
Substitution Reactions in Tetraazaporphyrins
Olga G. Khelevina
and
Natalija V. Chizhova
Ivanovo State University of Chemistry and Technology, Institute of Solution Chemistry of Russian Academy of Sciences, Ivanovo, Russia.
Peripheral modification of tetraazaporphyrins is an important approach for the synthesis of substituted tetraazaporphine derivatives, especially those which could not be obtained via direct template cyclotetramerization of maleic dinitriles and other similar synthons. This review presents the results of investigation of tetraazaporphine (I) and octaphenyltetraazaporphine (II) reactivity in substitution reactions at the pyrrole and benzene rings respectively (bromination, chlorination, chloromethylation, nitration and sulfonation) and nucleophilic substitution reactions of bromosubstituted tetraazaporphines.
Bromination of (I) by Br2 in glacial CH3COOH (20°C, 6 h) gives a 1:2 adduct of tetrabromotetraazaporphine with Br2, which undergoes a further transformation to tetrabromotetraazaporphine (III) by treatment with pyridine (yield 40%). Bromination of (I) by Br2 or NBS in pyridine gives dibromotetraazaporphine. Bromination of the benzene rings of (II) (Br2, CF3COOH, 20°C, 8 days) proceeds more slowly and gives octa(4-bromophenyl)tetraazaporphine (yield 71%).
Tetrachlorotetraazaporphine (IV) was obtained by heating a solution of (I) in SOCl2 at 45-550C for 6-8 h (yield 52%). The chlorination of (I) may be carried out also by PCl5 in acetic acid in the presence of H2SO4.
For (I) the sulfonation of the β-pyrrole positions of the macrocycle is possible. Using strong sulfonating reagents (oleum, gaseous SO3, HSO3Cl) it is possible to introduce from one to four and more SO3H groups. The tetrachlorosulfonic derivative of (I), which forms in HSO3Cl at 25°C (4 days), is easily hydrolyzed to the corresponding tetrasulfonic acid during isolation and purification (yield 25%).
Chloromethylation of (I) could be carried out by CH2O in HSO3Cl in the presence of NaCl at 20-25°C. Tetrachloromethyltetraazaporphine is hydrolyzed to tetrahydroxymethyltetraazaporphine during isolation and purification.
It is found that chloromethylation, sulfonation and sulfonylchlorination reactions of (II) proceed more readily compared of those of (I). Octa(4-sulfo-phenyl)tetraazaporphine, octa(4-sulfonychlorophenyl)tetraazaporphine and octa(4-chloromethylphenyl)tetraazaporphine were obtained by treatment of (II) with 100% H2SO4, HSO3Cl and CH2O in HSO3Cl respectively. Nitration of (II) with NaNO2 in CF3COOH (20°C, 10h) gives octa(4-nitrophenyl)tetraazaporphine (yield 50%).
High reactivity of (III) is observed in nucleophilic substitution reactions of bromine atoms. Zn-tetra(pyridiniumbromide)tetraazaporphine was prepared by heating of Zn-(III) in pyridine. Zn-tetrahydroxytetraazaporphine was synthesized by interaction of complex (III) with NaNO2 and K2CO3 in DMSO. Tetrafluorotetraazaporphine was obtained by heating of Mg-(III) with excess of KF in DMSO.
Modern State of Chemistry of Non-Traditional Classes of Coordination Compounds. Multi-Nuclear Complexes. Spheroids.
Boris D. Berezin
Institute of Solution Chemistry of Russian Academy of Sciences, Ivanovo, Russia
The modern state of chemistry on the whole and that of the chemistry of coordination compounds in particular is distinctive. Synthesis of more and more complicated molecular and super- (supra-) molecular systems is developing rapidly whereas investigating their fundamental properties, such as stability (kinetic, thermodynamic, thermal, photochemical), reactivity in the main types of interactions at a level of the study of mechanisms, is lagging disastrously. Such a situation can lead chemistry to a condition of uncertainty.
New, often unjustified concepts have appeared [
1]. As a result, boundaries and ideas of parts of chemistry such as coordination chemistry, bioinorganic and bioorganic chemistry, are diffuse. Unfounded terms, e.g. “supramolecular” coordination chemistry, photochemistry, electrochemistry, etc. are being introduced. In this connection it is necessary to consider the correlation of new parts and concepts of chemistry with strongly stated fundamentals of the main parts of chemistry, and coordination chemistry in particular.
Basic structural, kinetic and thermodynamic concepts of the chemistry of coordination compounds [
2,
3] of classic metal complexes, multi-nuclear complexes, clusters, rigid and nonrigid macrocyclic complexes (crown ethers, criptands, spheroids, speleands, cavitands, calixarenes, cyclophanes, etc.) are considered in the lecture. The natural tendency of coordination chemistry to spill over into the sphere of biology with the purpose of getting to know and saving animate and inanimate nature is shown [
4].
References
- Lehn, J.-M. Supramolecular Chemistry. VCH: N.Y., 1995; p. 334. [Google Scholar]
- Berezin, B. D. // Koordinatzionnaja Khimija. 1993; Volume V. 19, pp. 358–367. (in Russian) [Google Scholar]
- Berezin, B. D.; Berezin, D. B. Kurs sovremennoy organicheskoy khimii. Nauka: Moscow, 1999; p. 768. (in Russian) [Google Scholar]
- Abstracts of I International Conference on Porphyrins and Phthalocyanines. Dijon (France), 2000; p. 654.
Synthesis and Properties of Substituted Macroheterocyclic Compounds
Michael K. Islyaikin,
Elena A. Danilova,
Evgenii V. Kudrik,
Larisa D. Yagodarova,
Igor A. Yelkin
and
Rostislav P. Smirnov
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
The chemistry of the macroheterocyclic compounds (MCs) - analogues of phthalocyanine and porphyrazine - is now one of the most dynamic areas of research in Organic Chemistry. These compounds contain a core similar to that of phthalocyanine, which allows for a large variety of structural modifications. The various MCs are obtained by cyclisation of the diamines (A) and isoindoline or pyrroline derivatives (B) using stepwise or statistical condensation methods.
MCs and their metallocomplexes display interesting properties. The Cu-complex of the benzenetri(isoindole)macrocycle (ABBB-type) is used as a light and thermostabilizer of polyamide; Co-complexes are efficient catalysts for current sources, and various oxidation reactions. MCs have also interesting biological features.
The introduction of substituents into the macromolecules allows the modification of their properties, thus opening new perspectives for their applications. The combination of the large variety of core structures with the different nature and positions of substituents makes these compounds promising objectives.
Recent work shows that substituted MCs are favorable targets for the construction of organic molecular materials: they not only have interesting non-linear optical properties, but the capability to be organized in condensed phases such as liquid-crystals and LB-films.
Synthesis of MCs, particularities of their structure, characterization of the properties and perspectives of their chemistry are reviewed.
NMR Spectra of Porphyrins
Nugzar Zh. Mamardashvili
and
Oleg A. Golubchikov
Institute of Solution Chemistry of RAN, Ivanovo, Russia
The position of the proton signals in the 1H-NMR spectra of porphyrins is determined by the force of the ring π-electronic macrocyclic current. Under the shielding effects of macrocyclic ring currents the signals of NH-protons are observed in a very strong field: in the range from – 1.5 up to -4 p.p.m. The signals of β-CH- and ms-CH protons are observed in a weak field: ms-CH from 9.5 up to 10.5 p.p.m., and β-CH from 8.5 up to 9.5 p.p.m. The β-atoms of hydrogen of the pyrrole and pyrrolenine moieties are not equivalent, however at room temperature, fast tautomeric transformations take place on the NMR time scale and signals of the β-CH protons average out. In NMR spectra of protonated porphyrin the signals of NH-protons are displaced in a force field, and signals of the ms-CH and β-CH protons appear in a weak field. β-alkyl substitution of the porphyrin is accompanied by an insignificant displacement of NH-signals in a weak field and displacement of ms-CH protons signals in a strong one. The ms-substitution of the macrocycle results in a displacement of β-CH proton signals in a strong field and displacement of the NH-protons in a weak field.
The data summarized in the review convincingly proves that 1H-NMR spectra are useful for research into structural origins of porphyrins.
Acid-base Properties of Porphyrins
Vladimir G. Andrianov
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
Porphyrins (H2P) are a very interesting and unique class of tetrapyrrole compounds. Nowadays great success has been achieved in both porphyrin synthesis and the investigations of their physico-chemical properties.
Using non-aqueous solvents for the purposes of synthesis and practical applications of porphyrins and their metallocomplexes offers wide opportunities, if the thermodynamic constants of solvent autoprotolysis and acid-base dissociation constants of the H2P in these solvents are known. Ionization constants of H2P allow theoretical predictions of their reactivity with metal salts. Ionic forms of H2P play an important role in many chemical processes, photochemical ones in particular.
The ionization and acid-base properties of tetrapyrrolic compounds include the formation of anionic and cationic forms, i.e. processes that involve charge changes. Porphyrins can be considered as amphoteric compounds having both acidic (NH-acids) and basic (N-base) properties at the same time. Nitrogen atoms of imine type (-N=) are able accept surplus protons, whereas pyrrole type nitrogen atoms (N-H) are able to donate protons.
Data from investigations of the acid-base properties of H2P of different structural groups – chlorophyll, blood groups, tetrabenzo- and tetraphenyl- porphyrins, etc. in organic solvents of different nature are discussed in the lecture. The role of solvation processes in stabilization of ionic forms of H2P is shown. Correlation of acid-base properties of H2P with other physicochemical properties, coordination in particular, is carried out. Influence of functional substituents depending on their nature and position on the state of the nitrogen atoms of the reaction center is discussed.. Spectral characteristics of ionic forms of porphyrins are analyzed.
Anion-Cation Interactions of Water-Soluble Porphyrins in Solution
Nikolai N. Kruk,
Olga P. Parkhots
and
Nikolai V. Ivashin
Institute of Molecular and Atomic Physics, National Academy of Sciences, Minsk, Belarus
Among the large number of the synthetic porphyrin derivatives, the water-soluble ones are of particular interest. First of all, it is due to the greater potential for using them in industry, medicine and biology as drugs, catalysts, inhibitors and sensitizers for different reactions. The distribution of porphyrin in the human body, transport and accumulation in the tissues as well as the pathways and yields of the excitation energy deactivation depend on the microenvironmental properties such as polarity, hydrophobicity, ionic strength and others. Recently [
1], it was shown that cationic 5,10,15,20-tetrakis-(4-
N-methylpyridyl)-porphyrin undergoes shifts of both the electronic absorption and fluorescence spectra bands upon solvent changes. The number of lines in the porphyrin resonance Raman spectra revealed the same behaviour for the solvatochromic shift. This phenomenon has also been noted for other porphyrins, including anionic 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin. The observed pattern of the porphyrin response on the solvent change can not be explained with solvatochromic approaches.
The solution of this problem seems to be in the specificity of the water-soluble molecules that exist as the anion-cationic salts. Changes in the solvent leads to the different solvation ability for both porphyrin macrocycle and counterions (Cl-, I-, Na+ and others). In spite of the fact that anion-cationic interactions are of great importance for the elucidation of the spectroscopic and photophysical parameters of the water-soluble porphyrins, until now this question was not examined.
As a result due to the Coulombic forces the distance between the charged methylpyridyl groups and the counterions changes. Quantum-chemical calculations were invoked to study the problem. Upon decrease of the distance between counterion and porphyrin macrocycle the decrease in 0-0-transition energy is observed. The dependence of the energy of the 0-0-transition on the distance is expressed as 1/r2. The experimental data show good fit with this function. The distance has a maximum for highly polar solutions having high solvation ability. In the less polar solvent the porphyrin and counterion are likely to have a common solvation shell. The observed changes in the resonance Raman spectra are due to the changes in the force field due to the placement of the counterion in the proximity of the methylpyridyl substituents. As a result, the form of the vibration changes and the frequencies of the lines are changed. Thus, the extent of the counterion dissociation from the tetrapyrrolic macrocycle seems to govern the photophysical properties of the porphyrin moiety.
Upon interaction with biopolymers (proteins, oligopeptides, DNA, RNA) complex formation usually takes place. When complexed, a Coulombic interaction between the charged porphyrin macrocycle and the charged groups of the biopolymers occurs. In such a case changes in the photophysical and photochemical properties are observed. The contribution of solvatochromic, halochromic and heavy atom effects in the photophysical and photochemical property changes upon complex formation is discussed.
References
- Dzhagarov, B.M.; Kruk, N.N.; Chirvony, V.S.; Galievsky, V.A.; Turpin, P.-Y. Advances in Porphyrin Chemistry; Volume V.2. St-Petersburg University: SPb., 1999; pp. 50–69. [Google Scholar]
Polymer-Connected Porphyrins
Oskar I. Koifman
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia; e-mail:
Polymer-connected porphyrins, which include such important natural complexes as the haemoglobin found in blood and the cytochromes, represent a vast and unique group of intercomplex compounds that perform major biological, photochemical and fermentative functions in nature. The incorporation of metalloporphyrin molecules in polymer matrix causes improvements of the main properties of polymers and the appearance of new properties. At the same time, the rigid fixation of macrocyclic molecules in polymer chain causes the activation of the porphyrin’s properties. Owing to this fact, polymer-connected porphyrins are not only unique biopolymer models, but they also provide many opportunities for construction of effective catalysts for redox processes and new generation photosensitisers based on these compounds.
The following questions are discussed in this lecture:
- -
The classification of polymeric and polymer-bonding porphyrins and their specific properties as conditioned by bond type with polymeric matrix;
- -
Methods of polymer-connected porphyrin synthesis and porphyrin immobilization on polymeric matrix;
- -
Coordination properties of polymer-connected porphyrins in solution;
- -
Applied aspects and methods for development of the chemistry of polymer containing porphyrins.
Catalytic Systems Based on Immobilized Porphyrins
Anna B. Solovieva
Institute of Chemical Physics RAS, Moscow, Russia
The main approaches to designing catalytic systems based on metalloporphyrins and nonmetallic porphyrins immobilized on polymeric carriers are discussed. Considered are the different methods of immobilization result in the formation of both covalent-bound porphyrincontaining systems and systems with ionic, coordination, etc. bonds between porphyrins and a polymeric matrix. The basic types of processes catalyzed by porphyrins and metalloporphyrins are given. Discussed are the influence of a polymeric matrix as well as the methods for immobilizing porphyrins in a polymer matrix, the nature of the effect of the porphyrin ligand on the catalytic activity of bonded metalloporphyrins and porphyrins in different processes such as photo- and dark oxidation of biologically active hydrocarbons.
The problems involved in creating new effective porphyrin-containing polymeric catalysts based on the principles of how enzymes systems function in vivo are also considered.
Porphyrins in Ionometry
Galina I. Shumilova
and
S. M. Makarichev-Mikhailov
St-Petersburg state university, St.Petersburg, Russia
The application of metalloporphyrins as electroactive components (MAC) of the membranes of ion selective electrodes is very promising. The porphyrin ring system is very stable and exhibits an aromatic character. Almost all metals form stable intramolecular complexes with porphyrins. Membranes doped with metalloporphyrins have shown a potentiometric response to anions different from classical Hofmeister selectivity series [
1]. Now, electrode properties of a big number of metalloporphyrins have been extensively studied in order to understand the behavior of electrodes based on metalloporphyrins. Many authors have reported the influence of the nature of the central metal atoms on selective properties of the metalloporphyrin membranes [
2,
3,
4]. Many researchers argue that it is possible to create electrodes selective to one kind of ions by using porphyrins with different metals in the center of their structure. Another way of varying the properties of metalloporphyrin membranes is by addition of different substituents [
5,
6].
Solvent choice is important. The behavior of the metalloporphyrin membranes depends a lot on the pH of the studied solution. This fact limits the options for practical applications of the metalloporphyrins in ion selective electrodes. Much work has been devoted to the analytic uses of metalloporphyrin electrodes: Yoon et al. [
7] report using electrodes based on InOEP for the determination of choride-ion in blood serum. Sun et al. [
8] used electrodes with TPPS
4 for determination of iodide-ion in edible seaweed. Jain et al. [
9] described the possibility of designing an ISE based on using TPP as indicator electrode for potentiometric titration of Co
2+ with EDTA.
References
- Schulthess, P.; Ammann, D.; Simon, W.; Caderas, C.; Stepanek, R.; Kraudler, B. Helv. Chim. Acta 1984, 67, 1026.
- Steinle, E. D.; Bakker, T.; Sprichiger, U.E.; Pretsch, E. Anal. Sci. 1998, 14, 9.
- Gao, D. J. Anal. Chem. 1995, 351, 484.
- Steinle, E. D.; Schaller, U.; Meyerhoff, M.E. Anal. Sci. 1998, 14, 79.
- Gao, D.; Gu, J.; Yu, R. Q.; G. D. Analyst 1995, 120, 499.
- Badr, I.H.A.; Meyerhoff, M.E.; Hassan, S.S.M. Anal. Chim. Acta 1996, 321, 11.
- Yoon, J.; Shin, J.H.; Paeng, I.R.; Nam, H.; Cha, G.S.; Paeng, K.-J. Anal. Chim. 1998, 367, 1.
- Sun, C.; Zhao, J.; Xu, H.; Sun, H.; Zhang, X.; Shen, J. Talanta 1998, 46, 15. [PubMed]
- Jain, A.K.; Gupta, V.K.; Singh, L.P.; Khurana, U. Analyst 1997, 122, 583.
Porphyrin Containing Chemical Sensors
Alexander B. Valiotti
St-Petersburg State University, St. Petersburg, Russia
During the last ten years a lot of research has examined use of porphyrins, their metallocomplexes, phthalocyanines and other related compounds in chemical sensors. Sensors with various actions have been elaborated.
It is well known that various nitrogen containing macrocyclic compounds can be successfully applied as membrane active components of ion selective electrodes (a new class of ionophores), chemically sensitive layers of piezoresonators, optico-chemical sensors and others.
In recent times the idea of creating analytical arrangements imitating an animate “tongue” or “nose” has appeared in scientific practice. Attempts to construct such analytical systems, capable of recognizing complex composition of liquid media using a number of chemical sensors (so called “electron tongues”) as well as analytical systems for gas phase control (called “electron noses”) have been undertaken.
Obviously, porphyrins and their analogs could be used for creation of new multi sensor equipment. Some advantages in this area are presented in the review.
Liquid Crystal Crown Ethers
Olga B. Akopova
Laboratory of Liquid Crystals, Ivanovo State University, 153025, Ivanovo, Russia
The possibilities of synthesizing new molecular structures having macrocyclic polyethers properties (formation of "host - guest" complexes, ionophore activity, etc.) and capable of turning into the mesomorphous state (and consequently sensitive to small electrical, mechanical, thermal influences) has produced intensive efforts towards the synthesis of liquid crystal crown-ethers (LCCE).
The first information on LCCEs was published in the early eighties. Now about two hundred mesogenic molecular structures including fragments of CE (I-III) or relative macrocycles have been synthesized: calixarenes-IV, cyclophanes-II, azacrown - ethers - V and so forth. It amounts to less than 3 % of all synthesized liquid crystals, but the number of them will certainly grow.
The influence of the structure of crown-ethers and their analogues on the supramolecular architecture and properties (molecular design of nematic (I), smectic (II), columnar (III, IV) and cholesteric (chiral) (V) liquid crystals) is considered in this review.
The reported syntheses of liquid crystal crown-ethers with azomethine, amine and imine groups are analysed and ways of synthesizing macrocyclic polyethers (discotic mesogens) are proposed. Special attention is given to the prognosis of columnar mesomorphism of the compounds. The supramolecular structure of some of them, preparation and structure of LB films are considered. Some areas of possible liquid crystal CE application are discussed.
The work is accomplished with financial support of the Ministry of Higher Educational Institutions of the RF for the program "Scientific bases of methods of synthesis of chemical products and reagents”.
Heteroazocyclanes – Bifluorophores and Trifluorophores: Synthesis, Photophysical Properties, Non-Radiational Transfer of Energy of Electron Excitation in Macrocycles and on Lanthanides
S. A. Siling,
S. V. Shamshin,
N. V. Tokareva,
L. S. Lepnev
and
A. G. Vituhovskii
N. Nesmeyanov Institute of Organo-Element Compound RAS, Moscow, Russia; e-mail:
The syntheses of fluorescent macroheterocyclic compounds – hexazocyclanes – bifluorophores and trifluorophores are carried out. Hexazocyclanes – fluorophores were synthesized by condensation of dinitriles and aromatic diamines-fluorophores. Hexazocyclanes – bifluorophores are synthesized by condensation o-phthalodinitriles, containing heterocyclic substituents - donors of electron excitation energy and diamines-fluorophores - acceptors of energy of electron excitation. Hexazocyclanes – trifluorophores are synthesized by condensation of hexazocyclanes-bifluorophores with the salts of Eu and Sm. These compounds have a “sandwich” structure. Donor and acceptor of energy are chosen so that the field of radiation of the fluorophore-energy donor had a maximum overlap with the absorption field of fluorophore – energy acceptor.
The photophysical properties of macroheterocyclic compounds were investigated. It was established that in these compounds nonradiational transfer of energy of electron excitation takes place.
By using hexazocyclanes containing lanthanides it was shown for the first time in the world that excitation of luminescence of the lanthanide cannot be from the triplet level, but from the singlet level of the macrocyclic ligand-fluorophore. It was established that quantum yield of a solution of hexazocyclane-trifluorophore, that contains Sm reaches 85% at room temperature, when quantum yield of fluorescence of the salts of Sm and organic acids does not exceed 6-8%.
Physico-Chemical Problems of the Primary Events in Photosynthesis
Edward I. Zenkevich
Institute of Molecular & Atomic Physics, Minsk, Belarus
In recent years, on the basis of experimental methods (pico- and femtosecond spectroscopy, x-ray crystallography, single molecule spectroscopy, electron microscopy) and novel theoretical approaches combined with molecular modeling, serious success has been achieved in the investigation of structural peculiarities of natural photosynthetic systems in vivo (green plants and photosynthetic bacteria) as well as in the understanding primary photoevents in such objects (the electronic excitation energy migration in antennae light-harvesting complexes and the photoinduced charge separation in reaction centers).
In this report we would like to analyze the results that are of interest from physico-chemical and photophysical points of view: structural and energetic organization of antennae lightharvesting pigment-protein complexes of various types, mechanisms of interpigment interactions, the reaction center structure, the energy trapping by “special pair” and the mechanisms of the ultrafast photoinduced charge transfer with the quantum efficiency of ~100% at 293-77 К. The main peculiarities of primary photoprocesses obtained for native systems are compared with the results on the physico-chemical studies of structurally organized model complexes (on the basis of tetrapyrrolic compounds) possessing the effective and vectorial transformation of the light energy via the energy and/or electron transfer. The preparation of model multimolecular assemblies with functional properties in order to mimic important features of the energy and electron transfer photosynthetic events is one of the most popular tendencies of supramolecular photochemistry. The formation of the model systems is based on the simultaneous using of two principally different approaches (covalent bond between chromophores-reactants and non-covalent binding interactions of various nature). Using this strategy self-assembling nanoscale arrays have been prepared in solutions. Also prepared are thin polymeric films with structural and energetic parameters that are close to those for natural photosynthetic complexes (ring light-harvesting chlorophyll aggregates with coherent exciton transport, donor-acceptor systems with the photoinduced electron transfer in pico- and femtosecond time-scale at 293-77 K including long-distant, i.e., up to 15-20 Å charge separation). The results obtained for model systems of various types are compared with those known for native photosynthetic complexes.
Synthesis of Carboranylporphyrins and the Perspectives of Their Use for Boron Neutron Capture Therapy
Rima P. Evstigneeva
M.V. Lomonosov Moscow State Academy of Fine Chemical Technology, Moscow, Russia.
The development of the chemistry of carboranylporphyrins and their use for boron neutron capture therapy (BNCT) will be considered in this report. Synthesis of carboranylporphyrins is based on the use of several approaches for creation of their structure:
Rothemund reaction.
Acetylation of the amino groups of the porphyrin by chloroanhydrides of carborane acids.
A creation of amide bonds between the carboxylic groups of the porphyrin and the amino group of carborane.
Creation of ester bonds between the carboxylic group of porphyrin and the oxymethyl group of carborane.
Carborane joined to the aldehyde group of the porphyrin.
Besides the carboranyl group is σ-bonded to the porphyrin moiety of the molecule through the C-C or C-B bond.
The success of the different methods is estimated by quantitation of boron and the watersolubility of compounds, which is of prime importance for BNCT drugs. It is account the fundamental positions of BNCT-method and give the sample of carboranylporphyrins use for this purpose.
Experience of Introduction of Photogem, the First Domestically Produced Photosensitiser
Andrei F. Mironov
M.V. Lomonosov Moscow State Academy of Fine Chemical Technology, Moscow, Russia
Around the mid-eighties work began at our Academy on the creation of the first domestically produced photosensitiser for photodynamic therapy of cancer. The drug, that has subsequently been named Photogem, was obtained using original methodology based on natural hematoporphyrin. In 1992, clinical trials were started at the Hertzen Research Oncologic Institute and the Center of Laser Medicine. Later the Russian Oncologic Center joined these trials. The research carried out has demonstrated the good therapeutic effects of the drug and low side phototoxicity. In 1996, the Pharmocological Committee recommended Photogem for wide clinical use. In February 1999, by the order of the Minister of Public Health Services it was included in the list of drugs approved for medical use.
Nowadays Photogem is the only officially approved photosensitiser for PDT of cancer in Russia. More than 1500 patients have completed the course of treatment with Photogem. There results observed are 67% complete regression of tumors and 25% partial regression. At the early forms of cancer the positive effect is observed at 92 % cases.
Now we are conducting work to obtain permits for the use of Photogem in a number of countries in South-East Asia and South America.
Synthesis New Water-Soluble Derivatives of Pyropheophorbide a for Photodynamic Therapy of Cancer
Anna A. Axenova,
Yurii L. Sebjyakin
and
Andrei F. Mironov
M. V. Lomonosov State Academy of Fine Chemical Technology, Moscow, Russia
In the context of the design of second-degeneration photosensitizers for PDT based on natural porphyrins the synthesis new pyropheophorbide a derivatives has been performed. To increase the amphiphility of a molecule and improve of accumulation of sensitizer in tumors, we prepared substances containing different polar substituents in pyrrole ring A.
Introduction of additional polar substituents carried out by hydrobromination on vinyl group of pyropheoophorbide α by treatment with 50% HBr/AcOH resulted in the formation of the corresponding α–bromethylderivative and subsequent substitution of an alkoxy group for the bromine atom. Glycerol and galactose derivatives were selected as hydrophilic moieties for the syntheses. To increase the selectivity of alkylation, we used their isopropylidene derivatives. The yields were 63 % and 30% for the glyceryl and galactosyl derivatives, respectively.
For the preparation of galactosylpyropheophorbide we used also the method of activation of the α-hydroxyethyl group by transformation into trifluoroxyethylderivatives and condensation with the carbohydrate. Starting α-hydroxyethypyropheophorbide was synthesized by hydrolysis of the corresponding α-bromomethyl derivative. Overall yield in this case was 56%.
Deprotection with TFA/H2O (7:1) afforded water-soluble derivatives with different degrees of amphiphility that would allow studying how sensitizer penetration and accumulation in tumors depends on the number of hydroxyl groups.
The Synthesis of Novel Porphyrin-Quinone Systems
Elena A. Axenova
and
Andrei. F. Mironov
M. V. Lomonosov State Academy of Fine Chemical Technology, Moscow, Russia
These has been a great deal of recent research on molecular assemblies containing porphyrin for the modeling of various biochemical processes such as electron transport, energy transformation, metabolism of medicines, etc. One of the approaches for the study of photoinduced electron transfer is building in of molecules in Langmuir-Blodgett (LB) films. The aggregation of molecules in monolayers changes their photophysical characteristics and leads to the loss of efficiency of energy transformation. Therefore we used as donor a hindered 5,10,15,20-tetrakis-(3,5-di-tert-butylphenyl)porphyrin (TBP) that exhibits a low tendency to aggregate in LB-matrixes,.
The aim of our work was synthesis of porphyrin-quinones with two aldehyde groups. The starting 2-formyl-TBP was prepared by Vielsmeier reaction in high yield. The second starting material, 2,5-dimethoxybenzylamine, can be easily converted into quinone fragment by a demethylation reaction, followed by oxidation under mild conditions.
2-(2,5-dimethoxybenzylamino)methyl-TBP was obtained by the interaction of 2-formyl-TBP with 2,5-dimethoxybenzylamine and subsequent reduction of the generated Schiff’s base with sodium borohydride. The overall yield based on TBP was about 60%. The synthetic scheme for the synthesis of the intermediate amides containing two aldehyde groups included the following steps: condensation of 2-formyl-TBP with malonic acid and decarboxylation, transformation of the resulting 2-(2-carboxyvinyl)-TBP into chloroanhydride and, finally its treatment with 2,5-dimethoxybenzylamine. The overall yield of respective amide was about 50%. Mass-, UV-, IR- and NMR-spectra confirmed the structures of all compounds.
Tetraarylporphyrin-Labelled Poly(N-isopropyl-acrylamides)
Yurii S. Avlasevich,
Vladimir N. Knyukshto
and
Konstantin N. Solovyov
Institute of Molecular and Atomic Physics, National Academy of Sciences of Belarus, Minsk, Belarus
Polymers containing chromophore units are extensively synthesized and studied in connection with their diverse applications in science and technology. One of the important trends of the investigation of such polymers is the creation of molecular systems modeling biological objects [
1].
Specifically, in biophysical processes an important and, to a large extent, not yet elucidated role is played by conformational changes in biopolymers, in the first instance, proteins, including chromoproteins and pigment-protein complexes. The development of the generation of so-called "smart" polymers makes it possible to model such transformations and to control them by changing external factors, such as temperature. These polymers are using for biocatalysts immobilization, in sensors and for controlled drug release.
Owing to the exceptional role of tetrapyrrole pigments, chlorophyll and heme, in animate nature, polymers bearing porphyrinic units are of considerable interest as biomimetic models. For this reason, we are carrying out research in the field of synthesis and investigation of the "smart" polymers, derivatives of poly(N-isopropyl-acrylamide) (PNIPAM) containing porphyrin and metalloporphyrin units [
2,
3,
4,
5,
6,
7]. Polymers containing porphyrin moieties in their side chains can be prepared by the polymerisation of porphyrins containing vinyl groups or by reactions of prepolymers with porphyrin derivatives.
A series of methods of synthesis of tetraarylporphyrin-labelled poly(N-isopropylacrylamides) with various molecular masses and porphyrin content are reported. These include copolymerization of N-isopropylacrylamide with acrylic derivatives of tetraphenylporphyrin: 5-(4-acrylamidophenyl)-10,15,20-triphenyl-porphyrin [
2] and 5-(4-acryloyloxyphenyl)-10,15,20-triphenylporphyrin [
3]. Reactions of the polymer side chain active groups with porphyrin derivatives were also used for the preparation of porphyrin-labelled polymers. Several samples were prepared by the reactions of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin with N-oxysuccinimide containing prepolymer [
4] and by those of 5-(4-pyridyl)-10,15,20-tri(4-methoxyphenyl)porphyrin with poly(N-isopropylacrylamides) bearing bromoalkyl groups in their side chains [
5,
6].
In the spectra of the polymers obtained by copolymerization there appear several new bands. We attribute these bands to chlorin and bacteriochlorin structures which are obtained by the reaction of TPP moieties with radicals produced upon dissociation of AIBN. In the absorption and fluorescence spectra of Zn-porphyrin labelled PNIPAMs bacteriochlorin bands are especially pronounced [
5].
The spectra of Zn-porphyrin-labelled PNIPAMs obtained by quaternization have characteristic features. The absorption spectra of these objects are typical for ZnTPP, whereas fluorescence spectra show exciplex formation upon excitation. In solutions of polymer containing long-chain flexible spacer the exciplex appears both in methanol and in water. In solutions of polymer containing short-chain spacer the exciplex appears only in water.
Finally, we have reported that such polymers can be used for the creation of thermally controllable photophysical system [
7]. Fluorescence quantum yield in aqueous solution of one of the described polymers is quenched by six times in comparison with organic medium. Its heating results in phase separation, which is accompanied by fluorescence enhancement. The fluorescence quantum yield above LCST reaches its value for the dioxane solution. This process is thermally reversible and fluorescence is quenched again upon cooling below LCST.
References
- Tsuchida, E.; Nishide, H.; Yuasa, M. Macromolecules 1989, 22, 66–72.
- Avlasevich, Yu.S.; Kulinkovich, O.G.; Knyukshto, V.N.; Losev, A.P.; Solovyov, K.N. Polymer Science A 1997, 39, 1155–1162, Translated from Vysokomol.Soedin..A, 1997, 39, 1740-1748.
- Avlasevich, Yu.S.; Knyukshto, V.N.; Solovyov, K.N. Doklady of the NAS of Belarus 2000, 44, 56–59, (Russ.).
- Avlasevich, Yu.S.; Knyukshto, V.N.; Kulinkovich, O.G.; Solovyov, K.N. Zh. Prikl. Spektrosk. 2000, 67, 483–487, (Russ).
- Avlasevich, Yu.S.; Kulinkovich, O.G.; Knyukshto, V.N.; Solovyov, K.N. J. Appl. Spectr. 1999, 66, 597–601, Translated from Zh. Prikl. Spektrosk., 1999, 66, 538-541.
- Avlasevich, Yu.S.; Chevtchouk, T.A.; Knyukshto, V.N.; Kulinkovich, O.G.; Solovyov, K.N.J. Porphyrins and Phthalocyanines. 2000, 4. (in press).
- Knyukshto, V.N.; Avlasevich, Yu.S.; Kulinkovich, O.G.; Solovyov, K.N. J. Fluoresc. 1999, 9, 371–378.
Synthesis of New Platinum-Containing Phthalocyanine Complexes
Vladislav M. Mytsyk,
Viktor N. Nemykin,
Irina N. Tretyakova
and
Sergey V. Volkov
V.I.Vernadskii Institute of General and Inorganic Chemistry, Kiev, Ukraine
Phthalocyaninato metal complexes are effective second-generation photosensitizers for photodinamic antitumor therapy (PDT). On the other hand, the most popular antitumor drugs are the platinum-based complexes. For this reason the combination of photodinamic activity of the different photosensitizers and high cytotoxicity of the platinum-based complexes should lead to development of the new high-effective antitumor drugs. Tetrakis(3- pyridyloxy)phthalocyanines of zinc (1) and cobalt (2), and their derivatives with NH4[PtNH3Cl3] (3, 4), and cis-Pt[DMSO2Cl2] (5, 6) were obtained. Also the heteronuclear complexes consisting of oktacarboxy(phthalocyaninato)cobalt unit and “cis-platinum” in ratio PcCo(COOH)8 : “cis-Pt” from 1:1 to 1:4) (7-10) were synthesized.
All prepared complexes are the stable green (1, 3, 5) and blue (2, 4, 6, 7-10) substances. Compounds were characterized by IR, UV/Vis, MS, and NMR spectra. Composition of the synthesized complexes was confirmed by elemental analyses.
The aggregation effect of compound (1) was studied in various organic solvents such as DMSO, pyridine, 1,2,4-trichlorobenzene with pyridine; and the other compounds – in DMSO only. The solutions of (1, 5) had concentration 1 x 10-6 - 2.5 x 10-5 mole/l, the solutions of (2, 4, 6) had concentration 5x 10-7 - 1x 10-4 mole/l. In this case, Beer’s law is obeyed.
Dependence of complex formation on temperature was investigated for compound (1) with cis-Pt[DМSO2Cl2] and trans-Pd[DМSO2Cl2] at concentrations ranging from 1x10-6 to 1x10-3 mole/l. Thus, all obtained compounds are the high-effective drugs for photodynamic and dark antitumor therapy.
Synthesis and Photophysical Study of Diporphyrin Arrays with Peptide Spacers
Nadezhda V. Konovalova,
Valentina N. Luzgina
and
Rima P. Evstigneeva
M. V. Lomonosov State Academy of Fine Chemical Technology, Moscow, Russia
Synthetic porphyrins share many of the general structural, chemical, redox and photophysical features with the chlorophylls and bacteriochlorophylls of natural photosynthetic systems. A main difference is that porphyrins absorb strongly only in the blue while chlorins and bacteriochlorins are characterized by strong absorptions both in the blue and in the red regions of the solar spectrum. However, porphyrins due to their accessibility and stability are suitable for creation of model photosynthetic systems, which are able to reproduce at a simpler level some individual stages of natural photosynthesis.
Recent investigations have demonstrated that peptide-bridged diporphyrin arrays can be used for study of electronic couplings as well as energy and electron transfer between tetrapyrrole components of natural photosynthetic pigment-protein complexes. To continue our program aimed at developing of artificial photosynthetic systems, we have prepared a set of diporphyrin structures based on tetraphenylporphyrin derivatives bridged by peptide spacers including Gly, Val and Phe amino acids. Diporphyrins have been prepared by condensation of porphyrin derivatives of amino acids and peptides by the DCC-method. Structures of all synthesized compounds have been confirmed by UV-VIS, IR-, 1H-NMR-spectroscopy and mass-spectrometry.
Photophysical properties of diporphyrins have been studied by steady state and picosecond time-resolved fluorescence spectroscopy, and fluorescence quantum yields and lifetimes have been determined. The results of investigation show the possibility of intramolecular excitation energy transfer in synthesized compounds.
Synthesis and Properties of β-Diketone Phthalocyanine Complexes of Titanium, Zirconium and Hafnium.
Larisa A. Tomachynski,
Viktor Ya. Chernii
and
Sergey V. Volkov
V.I.Vernadskii Institute of General and Inorganic Chemistry, Kiev, Ukraine
Unsaturated phthalocyanine complexes of the transition metals have attracted considerable interest over the last few years. β-Diketone phthalocyanine complexes of titanium, zirconium and hafnium were obtained starting from dichloro-, oxo-, and dihydroxo-(phthalocyaninato)metal (IV) complexes (PcMCl2, PcM=O, and PcМ(OH)2, where М- Ti, Zr, Hf) at 110 – 1300C. When PcMCl2 was used as the initial compound the reaction was carried out with base.
Contrary to the starting materials, all prepared complexes pcm(β-dik)2, (β-dik – acac, fod, dpm) are highly soluble in various organic solvents such as toluene, chloroform, and others. all synthesized compounds are dark, blue-green, microcrystalline substances which are stable in solutions and air. complexes were characterized by ir, uv/vis, and nmr. 1h-nmr data of protons of the macrocycle and the axial substituents show cis-coordination of β-diketone ligands concerning of the macrocycle plane. chemical composition of all compounds is confirmed by the results of elemental analyses.
Synthesis of Water-Soluble Hemin Derivatives
G. P. Potapov
and
S. Z. Chatshihina
Siktivkar State University, Siktivkar, Russia
Earlier we have shown that salt-like products obtained from hemin after its treatment with organic bases can be dissolved in water. One more example of the preparation of a hydrophilic hemin compound is shown in this report:
Hydrophilic hemin derivatives are easily obtained during hemin treating with an organic free base – piperazine. Initial reagents hemin and piperazine were taken in 1:2 molar ratio. It is shown that formation of the salt bond between carboxy groups of hemin and the amino groups of piperazine is a simple and effective method of obtaining water-soluble hemin derivatives. Typical porphyrin macrocycle absorption bands are present in the electron absorption spectra of the synthetic products. Initial tests of physiological activity of compounds obtained are carried out.
Porphyrins and Chlorins with 1-Methyl-2-acetyl-3-oxobutyl-, 1-Methyl-3-oxobutyl-, and 1-Methyl-3-hydroxybutyl- Substituents: in vitro PDT Efficacy
Andrei V. Reshetnickov 1,
Olga Yu. Abakumova 1,
Tatyana A. Tsvetkova 1,
Artashes V. Karmenyan 2,
Aleksei G. Rebeko 3
and
Gelii V. Ponomarev 1
1
Russian Academy of Medical Sciences Institute of Biomedical Chemistry, Moscow, Russia;
2
N.N.Blokhin Cancer Research Center of Russian Academy of Medical Sciences, Moscow, Russia;
3
ChemBridge Corporation, Moscow Division, Moscow,Russia .
Having recently obtained a series of new porphyrin and chlorin derivatives (
I)-(
IV), we initiated an extensive structure-activity search, trying to make use of various chemical modification opportunities opened. Thus, we synthesized compounds (
V)-(
XII) [
1].
Photocytotoxicity and cytotoxicity of the PSs was determined using MTT test on PC12 cells with or without irradiation with 632 or 670 nm laser light at the doses of 20 J/cm2, with Al-sulfophthalocyanine. “Photosense” (Russia) and oligohemato-porphyrin-IX mixture "Photogem" (Russia) were used as reference drugs.
| Structure | Cytotoxicity%/control | Cytophototoxicity
CE50, М-6 | Structure | Cytotoxicity%/ control | Cytophototoxicity
CE50, М-6 |
|---|
| XII | 104*, 117** | 0.4 | III | 69*, 79** | 32 |
| VIII | 98*, 92** | 3 | VII | 72*, 79** | 32 |
| “Photosense” | 140*, 132** | 3 | I | 150*, 116** | 36 |
| “Photogem” | 150*, 170** | 4 | V | 135*, 110** | 40 |
| VI | 120*, 120** | 10 | IX | 118** | non active |
| II | 75*, 140** | 13 | X | 118** | non active |
| IV | 55*, 75** | 20 | XI | 120** | non active |
References:
- Reshetnickov, A.V.; Babushkina, T.A.; Kirillova, G.V.; Ponomarev, G.V. Khimiya Geterotsikl. Soed. (Engl. ed.: Chemistry of Heterocyclic Compounds) 2000, 3. in press.
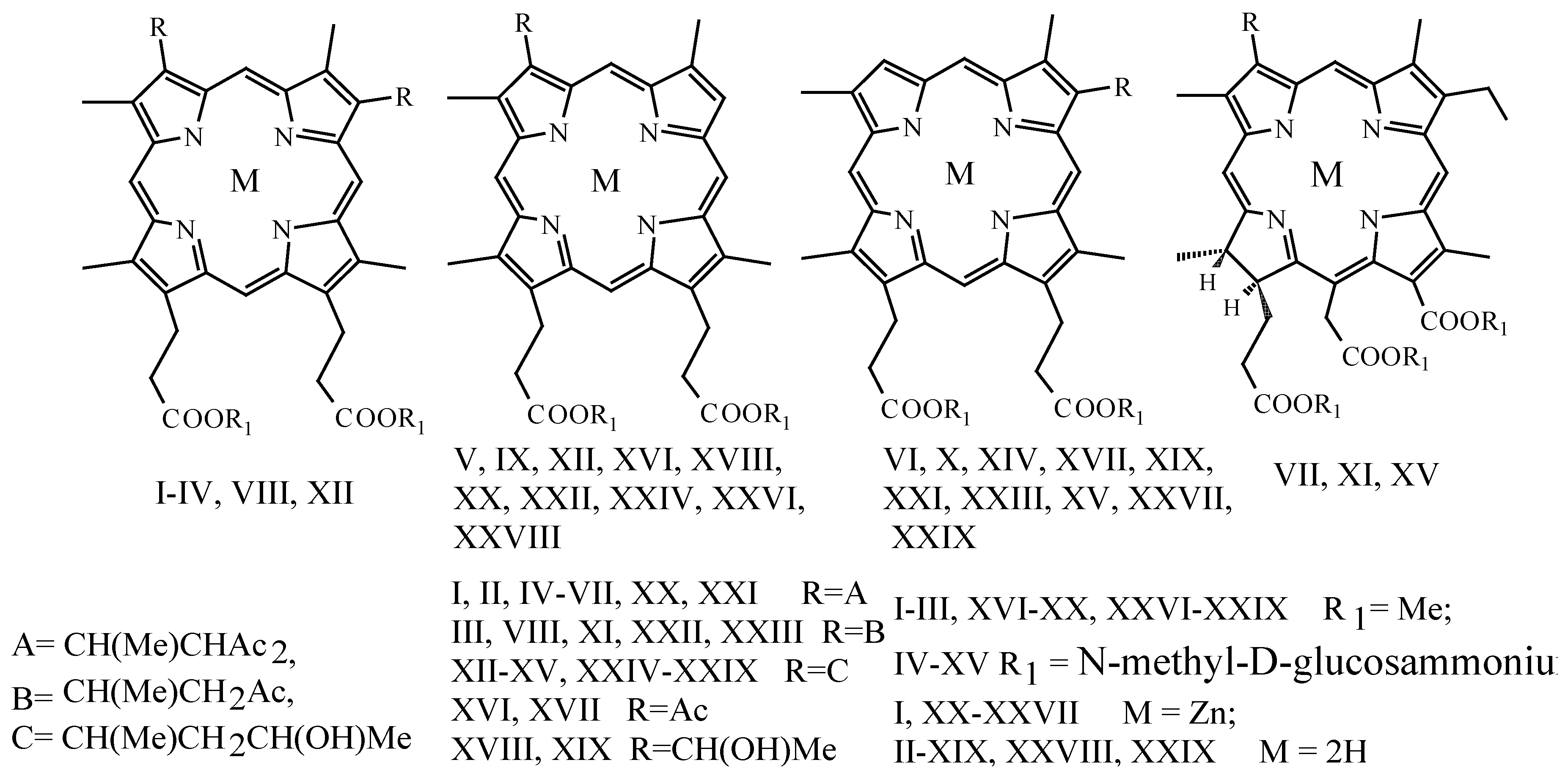
Synthesis and Chemistry of Porphyrins and Chlorins with 1-Methyl-2-acetyl-3-oxobutyl Substituents
Andrei V. Reshetnickov
and
Gelii V. Ponomarev
Russian Academy of Medical Sciences Institute of Biomedical Chemistry, Moscow, Russia
Taking to consideration previous syntheses [
1,
2] of compounds (
I,
II,
III), we have developed the chemistry and prepared a group of principally new amphyphilic water-soluble porphyrin- and chlorin-type photodynamic sensitizers (PSs) (
IV-
XV) (
Scheme 1).
The target PSs have been the acid hydrolysis products of derivatives prepared by borohydride reduction of known monoacetyl-porphyrins (
XVI,
XVII to
XVIII,
XIX), followed by the reaction with acetylacetone (
XX,
XXI) [
1], deacetylation (
XXII,
XXIII), reduction of the remaining carbonyl function (
XXIV,
XXV), esterification (
XXVI,
XXVII) and demetallation (
XXVIII,
XXIX).
Disubstituted porphyrin PS was obtained from hematoporphyrin-IX by the reaction with acetylacetone, followed by the above sequence of modifications. Chlorin PSs were derived from chlorin e6 through its hydrobromination, treatment with methanol and the same acetylacetone reaction, deacetylation and reduction sequence.
All water-soluble derivatives have been biologically tested on PC12 cells in both dark and light experiments.
References:
- Ponomarev, G.V.; Kirillova, G.V.; Shul’ga, A.M. Khimiya Geterotsikl. Soed. 1991, 11, 1564.
- Karnaukh, I.M.; Moskovkin, A.S.; Ponomarev, G.V. Khimiya Geterotsikl. Soed. 1993, 11, 1478.
4-Aryloxy-5-Nitrophthalodinitriles and Derived Octasubstituted Phthalocyanines
Anatolii E. Balakirev,
Vladimir E. Maizlish
and
Gennadii P. Shaposhnikov
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
In this work the synthesis and physico-chemical properties of 4-aryloxy-5-nitrophthalodinitriles and phthalocyanines based on these compounds have been described.
The initial phthalodinitriles (II-IV) have been obtained by the reaction of nucleophilic aromatic substitution of halogen atom in 4-bromo-5-nitrophthalodinitrile (I) with the use of substituted phenols (p-tert.-butyl; p-nitro; p-carboxy). The reaction was carried out at room temperature in the medium of DMF in the presence of triethylamine.
Octasubstituted metallphthalocyanines have been obtained by the interaction of synthesized phthalodinitriles with the acetates of corresponding metals at 200-210 oC.
Tetra-4-(p-tert.-butyl)phenoxytetra-5-nitrophthalocyanine (IId) has been obtained by the reprecipitation of corresponding magnesium complex from concentrated sulphuric acid.
Complexes (IIa-d) are soluble in chloroform, benzene, acetone, DMF and concentrated sulphuric acid. The introduction of electron withdrawing substituents at the p-position results in the disappearence of solubility of complexes (IIIa,b and IVa) in chloroform and benzene.
An influence of the nature of a substituent in the phenyl group on the position of Q-band of the electronic absorption spectra of synthesized complexes has been found.
This work was carried out with the financial support of a Ministry of Education grant in the field of Fundamental Research.
The Synthesis and Properties of Porphyrazines with Annelated Heterocyclic Fragments
Anatolii E. Balakirev,
Vladimir E. Maizlish
and
Gennadii P. Shaposhnikov
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
This report is devoted to the synthesis and physico-chemical properties of phthalodinitriles containing thiadiazole and triazole fragments and also cobalt complexes based on them.
4,5-Diaminophthalodinitrile (I) has been chosen as initial substance for the synthesis of phthalodinitriles with the condensed fragments of thiadiazole and triazole.
5,6-Dicyanobenzotriazole (II) has been obtained by the diazotation of 4,5-diaminophthalodinitrile with sodium nitrite in concentrated sulphuric acid at 0-5 oC.
The new approach was developed for the synthesis of 5,6-dicyanobenzothiadiazole (III), which was obtained by interaction (I) with thionylchloride at boiling the reaction mass during 5 h.
Cobalt porphyrazines have been obtained by the interaction of the synthesized phthalodinitriles (II, III) with cobalt acetate (II) in the presence of urea at 200-210 oC.
The nature of heterocyclic fragment influences greatly the physico-chemical properties of complexes. The presence of thiadiazole and triazole fragments gives the products solubility in aqueous solutions of mineral acid. Moreover the triazole fragment imparts solubility in alkaline aqueous solutions. These complexes are insoluble in water, chloroform, benzole, acetone, DMF (with the exception of triazole complex). They are soluble in concentrated sulphuric acid.
The introduction the heterocyclic fragments to a phthalocyanine molecule causes the batochromic shift of Q-band as compared with nonsubstituted cobalt phthalocyanine, the greatest shift being observed in the case of thiadiazolecontaining complex.
This work was carried out with the financial support of a Ministry of Education grant in the field of Fundamental Research.
Preparation of Chlorophyll Derivatives.
Dmitrii V. Belykh,
Ludmila P. Karmanova
and
Alexander V. Kutchin
Institute of Chemistry, Komi S.C., Uralian Division of RAS, Siktiyvkar.
Chlorophyll derivatives based on pheophorbides and purpurin 18 are used in the synthesis of photosensitizers for photodynamic therapy of different diseases, including oncologic ones.
We have isolated pheophorbides from the lipid fraction of substances extracted from medicinal plants and obtained chlorophyll derivatives derived from them:
Pheophorbides (4,5) and ethyl pheophorbides (2,3) (a)- and (b)-series are contained in the lipid fractions. They are converted into the corresponding methyl pheophorbides by boiling in a 5%-solution of concentrated sulfuric acid in methanol. The mixture of methyl pheophorbides is fractionated by column chromatography and pure methyl pheophorbides (a) and (b) are isolated. It is established that besides these compounds methyl pheophorbides (a') and (b') and also methyl 10-hydroxy derivatives (9) and (10) are present in the mixtures. Compounds (1) and (6) are converted into methyl pyropheophorbides (11) and (12) by decarboxylation. Purpurin 18 (13) is obtained by oxidizing a mixture of (1,6-8) in 0,5% solution of KOH in ethanol.
Analysis of reaction products is carried out by TLC and HPLC. Structures of isolated compounds is confirmed by 1H-NMR, 13C-NMR, IR- and UV-VIS-spectroscopy.
Changes in Acidic and Basic Properties of Porphyrin Macrocycles upon Introduction of Different Substituents on the Molecule
Tatiana V. Gromova,
Tatiana V. Karmanova
and
Boris D. Berezin
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
Substituents in the porphyrin molecule influence considerably its reactivity. Interactions of 2,3,7,8,12,13,17,18-octabromo-5,10,15,20-tetraphenylporphyrin (I), 2,3,7,8,12,13,17,18-octamethyl-5,10,15,20-tetraphenylporphyrin (II) and dodeca-phenylporphyrin (III) with acetic acid and bases (L), such as pyridine (Py), piperidine (pip) dimethylformamide (DMF), dimethylsulfoxide (DMSO) were studied spectrophotometrically.
Batochromic shifts of Soret bands (B) are observed in toluene for porphyrins (
I-III), and bands in the visible region are weaker and wider as compared with unsubstituted tetraphenylporphyrin. During titration of porphyrin (
I) with pip in toluene an intensive maximum at λ=775 nm appears while bands
II and
III disappear. These changes may be regarded as resulting from changing symmetry of the macrocycle from D
2h to D
4h as a result of acid-basic interactions with L. Similar acid-basic interactions have been found earlier for phtalocyanines and tetraazaporphyrins [
1]. For porphyrins themselves such interactions with organic bases have been found for the first time. Equilibrium constants of these processes were measured. Two main reasons are supposed to explain an unusual behavior of porphyrins mentioned above. These are conformational distortion of macrocycle and electronic influence of substituents.
It is found that porphyrin III is more stable in the form of acid monocation H3DPP+ in comparison with the neutral form H2DPP. The equilibrium constant for this reaction is determined to be: H3DPP+ + L ↔ H2DPP + LH+.
References
- Whalleg, M. Conjugated Macrocycles. Part XXXII. Absorption spectra of tetraazaporphyrins and phatalocyanines. Formation of pyridine salts. J. Chem. Soc. II 1961, 866–869. [Google Scholar] [CrossRef]
Physico-Chemical Properties of Linear Di- and Tetrapyrrole Compounds
Galina B. Guseva
*,
Elena V. Antina
and
Michael B. Berezin
*
State University of Chemistry and Technology, Institute of Solution Chemistry RAS, Ivanovo, Russia
It is presently stated that the linear polypyrrole compounds are the general metabolites for all natural cyclic tetrapyrrole pigments (porphyrins, chlorins, cobalamins), playing an extremely important role in the animal and plant organisms. The linear di- and tetrapyrrole compounds are also widely used in preparative organic chemistry for the synthesis of different synthetic and natural porphyrins. That is why the information about the reactivity and the other physico-chemical properties of the compounds of this class is of great importance.
In the present paper the influence of the solvent nature and the solute concentration on the state of 3,3’,5,5’-tetramethyl-4,4’-dibutyldipyrrolylmethene, 2,2’,1,3,7,17,19-hexamethyl-2,8,12,15-tetrabutylbiladiene-ac and its salts with HBr in solutions of n-propanol, benzene, chloroform, pyridine, DMF and DMSO were studied by spectrophotometry. It is determined that in the electron donor solvents as well as in the presence of the nucleophilic reagents (NH3, sodium propylate) deprotonation processes of the linear polypyrrole hydrobromides are observed. The partial destruction was registered at these conditions in the biladiene-ac solutions. The rate and extent of the process course depend on the electron donor solvent properties, solution concentration and chromophore molecule self association.
Cu2+, Ni2+, Co2+, Zn2+ and Hg2+ complexes with 3,3’,5,5’-tetramethyl-4,4’-dibutyldipyrrolylmethene are synthesized for the first time. The state of the compounds in the n-propanol, benzene, chloroform, DMF and pyridine solutions is characterized by their electron absorption spectra. The stoichiometric complex composition is defined by the molar ratio method. The influence of the solvent and metal nature on the spectral characteristics of dipyrrolylmethene complexes solutions are evaluated.
It is determined that metal ions have an auxochrome effect on ligand chromophore in metallocomplex structure, which increases in the following series of ions: Zn2+ ≤ Hg2+ ≤ Co2+ < Ni2+ < Cu2+. The peculiarities of the metallocomplex thermooxidation destruction are studied by the method of thermogravimetry. The composition of metallocomplexes in crystals is defined. On the derivatogrammes of copper, cobalt and mercury complexes in the temperature range of 130-160°C the presence of conformational rearrangements for which the enthalpy changes are calculated is noted.
Regularities of Extracoordination of Nitrogen Containing Ligands by Ga3+-, In3+-, Tl3+-Tetraphenylporphine Complexes
Svetlana V. Zaitseva
*,
Sergei A. Zdanovich
*
and
Tatiana A. Ageeva
*
Institute of Solutions Chemistry of Russian Academy of Sciences,
**
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
**
The thermodynamic characteristics of stability of metalloporphyrin extracomplexes are rather important for interpreting their physico-chemical properties and predicting technological processes. Growing interest in porphyrins with multicharged metal atoms is connected, first of all, with the search for effective catalysts for redox processes. Compounds perform their catalytic activity in the structural form of extracomplexes. We investigated coordination properties of tetraphenylporphine complexes of metals of a subgroup of aluminium (Ga -, In -, Tl-) with various acido-ligand and extraligands in chloroform. On the basis of both experimental and calculated data it is established the correlation between stability (Ks) of extracomplexes (X)M(L)TPP (M = Ga3+, In3+, Tl3+ L = N-methylimidazole, imidazole, pyridine, dimethylformamide) and basicity of additional molecular ligand, nature of central metal atom and acidoligand X. Кs values are in good correlation with calculated values of energy of M-L bonds. Using ZINDO1 calculation geometrical and energy parameters of Ga-, In-porphyrins and their extracomplexes were obtained.
Study of Axial Coordination Reactions of Manganese Porphyrins with Different Porphyrin Macrocycle Structure.
Tatiana V. Karmanova,
Tatiana V. Gromova,
Irina B. Zadorova
and
Boris D. Berezin
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
Many useful properties of porphyrins result from peculiarities of their structure. As a rule the porphyrins perform their biological and catalytic functions as metallocomplexes. Many metalloporphyrins are able to coordinate additional ligands that are known as extra ligands.
2,7,12,17-tetrabromo-5,10,15,20-tetraphenylporphyrin (I), octaphenyltetra-azaporphyrin (II) and their complexes with Mn(d4) were synthesized. The influence of the porphyrin macrocycle structure on extra coordination reaction with organic bases in toluene was studied using UV-visible spectroscopy.
The following coordinating solvents were chosen: pyridine (Py), piperidine (pip) dimethylformamide (DMF), dimethylsulfoxide (DMSO).
In the presence of piperidine Mn(d4)P → Mn(d5)P reduction occurs. For porphyrin I the first step involves the addition of piperidine molecule to the sixth coordination position with subsequently immediate reduction of Mn(III) to Mn(II) and substitution of acidoligand (Ac-) by pip. The two steps are spectrally different. The bromine atoms possess dual electronic nature,namely the positive conjugation effect (+C) and the negative inductive effect (-I). The combined influence of bromine atoms in β-positions and phenyl groups in meso-positions results in distortion of macrocycle molecule, and also influences the coordination center state.
Upon addition of pyridine to manganese porphyrin I the substituon of (Ac-) by Py molecule occurs. In the case of DMSO for porphyrin I the substitution of (Ac-) by DMSO is observed (thermodynamic process), with subsequent reduction of Mn(III) to Mn(II) (kinetic process).
The octaphenyltetraazaporphyrin macrocycle includes nitrogen heteroatoms in its conjugated system of π-bonds. The electronic density in the azaporphyrin macrocycle is substantially higher than that in porphyrins. For porphyrin II an interaction with pip is a kinetic process. The reaction goes under conditions of considerable excess of pip. The kinetic studies have showed, that the reaction of Mn(III)P with pip is described by an equation of the first order on porphyrin. Low Kv values and zero reaction order on pip extra-ligand evidences that kinetic process observed by us in UV-visible spectra may be regarded to acetate ion-piperidine molecule substitution process.
Under titration by Py and DMSO slight changes of charge transition band occur in electronic absorption spectra of porphyrin II. The substitution of (Ac-) by Py or DMSO molecules almost does not influence on electronic density distribution on MO of manganese porphyrin macrocycle and geometric parameters of coordination center and, therefore, does not appear in electronic spectra.
Electrochemical Properties of Cobalt Complex of 5-aza-2,3,7,8,12,18-hexamethyl-13,17-dibutylporphyrine
Marina V. Kurach,
Michael I. Bazanov
and
Alezxander S. Semeykin
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia; e-mail:
The use of porphyrin complexes as biologically active substances and medicines is current. In some cases their functional activity depends on the ability of the complexes to molecular oxygen adsorption and possibility of their participation in oxidation-reduction processes. Relying on this the study of oxidation-reduction behavior of the porphyrin compounds can give new information and develop the ways to clarify of the mechanism of its catalytic or inhibitory action in the real processes.
In present paper the results of investigation of cobalt complex of 5-aza-2,3,7,8,12,18-hexamethyl-13,17-dibutylporphyrin using the cyclic voltammetry method is shown. The study was made on the model porous electrode in 0,1 M KOH solution in the atmosphere of argon and oxygen.
It is determined that in the studied potential range from +0,5 to -1,4 voltage the porphyrin complex can exist both in monocation and in different anion forms. The general scheme of the electrochemical transformations shows that both central ion and porpyrin ligand can be the site of electron localization.
The oxidation-reduction potentials of the electrochemical transformations under study were determined. The complex shows electrocatalytic activity in the process of the molecular oxygen reduction, the process goes mainly as 4-electron reaction.
Stability of Al(III) and In(III) Tetraphenylporphyrin Acidocomplexes from High Temperature Mass-Spectrometry
Olga V. Molodkina*,
Olga V. Pilipets
and
Tatiana N. Lomova
Institute of Solution Chemistry, Russian Academy of Science, Ivanovo Russia
Complexes of meso-tetraphenylporphine with Al(III) and In(III) bearing chloride or hydroxide as axial ligands ((X)MTPP, M = Al, In, X = Cl, OH) have been studied using electron impact mass-specrometry method. In the mass-spectra of all studied compounds the molecular ion peaks have been observed, which is indicative of the stability of complexes towards the electron impact process.
It is shown that the general feature of the defragmentation of Al(III)- and In(III)- tetraphenylporphyrins is the elimination of axial acido-ligand from the molecular ion [(X)MTPP]+· leading to the formation of [MTPP]+.
Such a process of dissociative ionization is confirmed by the presence of the cluster of the metastable ions at m* ≈ m2[MTPP]+/m[(X)MTPP]+ and by comparison of experimental and theoretical isotope distribution pattern.
Along with peaks of the mono-charged ions the mass-spectra of the complexes under investigation contain the peaks of double-charged ions [MTPP]++, and in the case of (Cl)InTPP, also [(Cl)InTPP]++. The characteristic feature of the mass-spectrum of (HO)AlTPP is the presence of the peak with m/z 1294, which is indicative about the formation of the μ- oxodimer (TPP)Al-O-Al(TPP).
The contribution of the M-N and M-X bonding to the stability of Al(III) and In(III) complexes of tetraphenylporphyrins has been elucidated on the basis of comparison of the relative intensities of the peaks of molecular and double charged ions in the mass-spectra with previously obtained quantitative data [
1,
2] on the solvo-protolitic dissociation of M-N bonds in metalloporphyrins.
The temperature dependence of mass-spectra pattern has been also investigated and thermodynamic parameters of the sublimation of metalloporphyrins have been determined.
References
- Lomova, T. N.; Zaitseva, S. V.; Molodkina, O. V.; Ageeva, T. A. Koord. Khim. 1999, 25, 424.
- Lomova, T. N.; Mozhzhukhina, E. G.; Shormanova, L. P.; Berezin, B. D. Zh. Obshch. Khim. 1989, 59, 2317.
Synthesis and Properties of New Unsymmetrically Substituted Phthalocyanines and Porphyrazines.
Ivan U. Nikolaev,
Evgeny V. Kudrik
and
Gennady P. Shaposhnikov
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
Unsymmetrically substituted phthalocyanines (Pcs) draw much attention due to their possible applications in non-linear optics, molecular electronics, etc.
This poster is devoted to the synthesis, investigation and spectral properties of new unsymmetrically substituted phthalocyanines and porphirazines, containing both donating and accepting groups.
Peripherally functionalized Pcs of the type M[PcAnB4-n] where n=1 to 4 were synthesized using different statistical condensation methods. Phthalocyanines, containing both 1,4-alcoxyphenyl- and phenyl- (or ditiacyclohexyl)- groups were synthesized by traditional mixed condensation, using Li 1-pentylate, or Mg 1-pentylate as template agents. Phthalocyanines of AB3, AABB and ABAB types, containing both strong accepting nitro- and strong donating 1,4-decyloxygroups, were obtained using statistical condensation of 4-nitrophtalimide and 3,6-didecyloxyphthalodinitrile in 9:1 molar ratio in the presence of urea, catalytic quantities of ammonium molybdate and copper (II) acetate and separated by column chromatography. Structures of new Pcs have been determined by CHN analysis, UV-VIS, NMR and massspectroscopy data.
The unsymmetrical substitution influence on spectral and physicochemical properties of new Pcs is discussed.
Immobilization of Synthetic Porphyrins on Polymers
Olga I. Nikolaeva,
Tatiana A. Ageeva
and
Oskar I. Koifman
Ivanovo State University of Chemical Technology, Ivanovo, Russia
One of methods used to produce polymer-bounded porphyrins is an immobilization of biologically active tetrapyrrolic macrocycles on polymer matrixes.
The method is practical because it allows using a series of polymers with already known and assigned properties, such as solubility, molecular weight, molecular-weight distribution, etc. This way also allows changing a structure of porphyrin fragment. To perform this kind of immobilization the porphyrins and polymer carriers have to contain active groups on the periphery of a molecule.
Poly-(styrene-co-allylalcohol) with the ratio of styrene/allylalcohol 70:30, 85:15, 95:5 in moles, obtained by suspension copolymerisation, was chosen as polymer carrier.
Synthetic porphyrins used contained –NH2, or -OH, or -COOH groups on the periphery of macroheterocycle, that allowed us to carry out synthesis of polymer-bounded porphyrins by immobilization of these porphyrins on synthetic polymer carriers with active hydroxyl groups.
With the aim of activation of polymer carrier it was modified by means of epoxy activation. Obtained compounds were characterized by their electronic absorption spectra in various solvents. The degree of immobilization of a porphyrin was determined spectrophotometrically. The content of porphyrin in its copolymer was from 1 to 3 mass. %.
Due to covalence bonding with a polymer carrier it was shown that polymer-bounded porphyrin take on an ability to dissolve in solvents, in which free porphyrin is insoluble, but the basic properties of the porphyrin are retained. The kinetic constants and thermodynamic parameters were determined. Findings about effect of polymer matrix on reactivity of polymerbounded porphyrin were made.
This work was performed with financial support of the Ministry of Higher Education of the Russian Federation (grant N 98-8-3.1-244).
Synthesis and Properties of β-octaphenyl Porphyrins with Different Degree of meso-Phenyl Substitution
ViktorijaV. L’vova,
Pavel A. Shatunov
and
Alexander S. Semeikin
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
A series of β-octaphenyl porphyrines with different number and localization of meso-phenyl substituents were synthesized via mixed condensation of 3,4-diphenylpyrrole and 2-hydroxymetyl-3,4-diphenylpyrrole with benzaldehyde. 2-Hydroxymethyl-3,4-diphenylpyrrole was obtained by sodium borohydride reduction of 2-formyl-3,4-diphenylpyrrole produced from 3,4-diphenylpyrrole by means of a Vilsmeier reaction.
The synthesis in methanol/HBr media at room temperature with intermediate porphyrinogen oxidation resulted in traces of desired products together with large amounts of difficult to separate impurities. The same conditions, but under reflux, gave compounds (I), (III-V). The use of boiling xylene/chloroacetic acid with passing oxygen through the reaction mixture afforded porphyrins (I), (III-VI). Surprisingly porphyrin (II) was not separated from the reaction mixture in both cases. The yield distributions of products obtained in these two syntheses were different.
In the series of porphyrins (I-VI) there is an increase of the degree of macrocycle distortion, which is reflected, for instance, in the growing bathochromical shifts all of absorption maxima in UV-vis spectra. The purity and identity of all compounds produced were confirmed by NMR, UV-vis spectral and TLC analyses.
Influence of Macrocyclic Ligand Structure on the Kinetic Stability of Magnesium Complexes.
O.V. Shukhto,
D.B. Berezin
and
B.D. Berezin
Ivanovo Solution Chemistry Institute of Russian Academy of Science. Ivanovo Russia
Classic porphyrins - porphine and its β - alkylderivatives have unrigid aromatic structures. Benzosubstitution and bridge N - substitution increase the rigidity and aromaticity of the macrocycle. Benzosubstitution in porphyrin ligands stabilizes ionic complexes strongly. Azasubstitution stabilizes magnesium complexes of porphyrins approximately ten-fold. Therefore, the investigation of β- substituents’ influence on the structure of the phthalocyanine core and as a consequence on the kinetic stability of unsubstituted phthalocyanines and their magnesium complexes is of interest.
We have investigated by spectrophotometric methods the process of solvoprotolitic dissociation of 4–tert-butylphthalocyanine and its magnesium complex. The rate constants of the reaction and energetic characteristics have been determined. As it was found, β- substituted complexes of magnesium phthalocyanine dissociates slower then unsubstituted ones under similar conditions. The literature data on the stability of porphyrins magnesium complexes of different structure in media on the basis of sulfuric and acetic acids have been systematized. The influence of β - substitution on the kinetic stability of magnesium complexes of porphyrins and phthalocyanines is shown.
Synthesis of New Triazoleporphyrazines
B. Cabezón 1,
S. Esperanza a,
M. K. Islyaikin 2,
E. V. Kudrik 2,
M. Nicolau 1,
M. S. Rodríguez-Morgade 1,
G. de la Torre 1,
I. A. Yelkin 2
and
T. Torres 1
1
Departamento de Química Orgánica (C-I), Universidad Autónoma de Madrid, 28049-Madrid, Spain
2
Department of Fine Organic Synthesis, State University of Chemistry and Technology, Ivanovo, Russia
Triazolephthalocyanines [
1] are phthalocyanine analogues in which one of the isoindole subunits has been formally replaced by the polar 1,2,4-triazole moiety, leading to macrocycles isoelectronic to phthalocyanines. We have described their synthesis [
1], deposition in Langmuir films [
2], as well as their conductive [
3] and non-linear optical properties [
4]. However, until now only nickel and copper-metallated compounds have been available in good yields through the reported procedures [
1]. On the other hand, the design of tailor-made triazolephthalocyanines for particular purposes demands the variation of different physicalchemical parameters by conventional organic synthesis, and the organization of the compounds at a supramolecular level.
Hence, we report on the preparation of free-base triazolephthalocyanines 1 via statistical condensation of the corresponding iminoisoindolines with 1,2,4-triazole in a non alcoholic solvent, like butyronitrile. Further metallation of these new macrocycles gives rise to metallotriazolephthalocyanines that are not accessible through direct template macrocyclization reaction.
Furthermore, we are also developing the preparation of related triazoleazaporphyrins of type 2 with increasing solubility in organic solvents. In this case, functional groups directly attached to the β-positions of the pyrrole subunits should have stronger coupling to the macrocycle core, thus allowing a fine tuning of their electric and optical properties.
References
- Cabezón, B.; Rodríguez-Morgade, S.; Torres, T. J. Org. Chem. 1995, 60, 1872. Fernández-Lázaro, F.; Sastre, A.; Torres, T. J. Chem. Soc., Chem. Commun.. 1994, 1525.
- Armand, F.; Cabezón, B.; Martínez-Díaz, M. V.; Ruaudel-Teixier, A.; Torres, T. J. Mater. Chem. 1997, 7, 1741.
- Armand, F.; Cabezón, B.; Araspi, O.; Barraud, A.; Torres, T. Synth. Met. 1997, 84, 879.
- Cabezón, B.; Fernández-Lázaro, F.; Martínez-Díaz, M. V.; Rodríguez-Morgade, S.; Sastre, A.; Torres, T. Synth. Met. 1995, 71, 2289.
Synthesis of σ-Aryl(alkyl)complexes of Fe(III)octaphenyltetraazaporphin and Investigation of their Interactions with N-Bases.
Olga V. Mal’chugina,
Pavel A. Stuzhin,
Tatiana M. Kruchinina
and
Boris D. Berezin
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
It is known that deactivation of catalytic hemoproteins under action of arylhydrazines proceeds due to the formation of Fe(III) σ-aryl complexes followed by transformation into Naryl complexes of Fe(II) in the presence of oxygen [
1].
The synthesis of pentacoordinated complexes of σ-aryl(alkyl) Fe(III)-octaphenyltetraazaporphin (R)Fe
IIIOPTAP (R= p-CH
3Ph, p-CH
3OPh, n-But) by interaction of initial complex (Cl)Fe
IIIOPTAP with corresponding Grinuar reactive according to method suggested in [
2] is reported here. However, the alkyl complex (n-But)Fe
IIIOPTAP could not be isolated in solid because of its instability in the presence of air oxygen in contrast to aryl complexes. The UV-VIS spectra shape of obtained compounds is typical one of low spin Fe(III) complexes.
The interaction of pentacoordinated σ-aryl complexes with pyridine derivatives L (L=Py, 3,4-Me
2Py, 3-ClPy) as well as 1-MeIm, Pip and BzNH
2 leads to the formation of hexacoordinated complexes (L)(R)Fe
IIIOPTAP according to equation (1). This was proved by spectrophotometric titration, the concentration constants of equilibrium in N-base extracoordination reaction were determined.
The stability of (L)(R)Fe
IIIOPTAP extracomplexes is increased with growth of σ-donor properties of N-bases. The σ- and π-interaction of aryl anions with Fe(III) ion may have an influence on the stability of these complexes as well. Depending on the nature of the extraligand in the trans-position the aryl anions may exhibit either π-donor or π-acceptor properties [
3]. The stability of complexes is decreased in the order (p-CH
3OPh)Fe
IIIOPTAP > (p-CH
3Ph)Fe
IIIOPTAP > (Ph)Fe
IIIOPTAP due to the weakening σ-donor properties of the aryl ligand.
References
- Ortiz de Montellano, P.R.; Kunze, K.L. J. Am. Chem. Soc. 1981, 103, 6534.
- Stuzhin, P.A.; Mal chugina, O.V.; Wolowiec, S.; Latos-Grazynski, L.; Berezin, B.D. J.Mendeleev Commun. 1998, 143.
- Stuzhin, P.A.; Mal’chugina, O.V.; Kruchinina, T.M.; Migalova, I.S.; Berezin, B.D. Jurn. Koordin. Khimii 1999, 25, 121.
Spectrophotometrical Investigation of Reaction of (Acetate) chromium (III)-tetraphenylporphin with Pyridine
Marina Yu. Tipugina
and
Tatiana N. Lomova
Institute of Solution Chemistry, Russian Academy of Science, Ivanovo, Russia
(Acetate)chromium(III)tetraphenylporphin complex (OAc)CrTPP was synthesized by reaction of H2TPP with Cr(OAc)3 in boiling benzonitrile with further evaporation of the solvent under vacuum. The complex was purified by column chromatography on Al2O3 with chloroform elution and characterized by UV-Vis and IR spectra.
The reaction equilibrium of (OAc)CrTPP with Py in toluene was studied spectrophotometrically over a wide region of Py concentrations at 298K (9.92
.10
-6 - 1.59 mole/l). Two steps were discovered for this reaction that follows from the form of titration curves. Equilibrium constants for both steps of the reaction were determined as 4
.10
2 and 2.8 l/mole respectively.
The tg of right pitch angle in lg((AP-Ao)/(A∞-AP)) - lgCoL coordinates was at first time found out to be 1/2. The formation of associate of coordinated and noncoordinated molecules of metalloporphyrins is confirmed.
The equilibrium (1) allows the determination of the rate of direct reaction by the spectrophotometrical method. The kinetics of the direct equilibrium reaction was studied at 298K and at Py concentration (0.19-1.98)
.10
-2 mol/l. The rate law for present reaction was found:
Values of k1 and k-1 are equal 11.8 min-1mol-1/2.l1/2 and 2.95.10-2 min-1mol1/2.l-1/2 respectively. The equality of values of the order (1/2) and of the equilibrium (1) molecularity confirms the supposition about interaction of extracomplex (OAc)(Py)CrTPP with noncoordinated molecule (OAc)CrTPP owing to pπ (Py) → dπ (Cr) interaction.
Protonation and Dissociation Reactions of Halogen-Nitrophthalocyanine Complexes with Copper(II) and Aluminium(III)
Elena E. Suslova,
Tatjana N. Sokolova,
Tatjana N. Lomova,
Svetlana V. Zaitzeva
and
Sergey A. Zdanovich
Institute of Non-aqueous Solution Chemistry of the Russian Academy of Sciences, Ivanovo, Russia
Earlier we have investigated the forms and reactivity of substituted with Cl- or COOH- groups copper(II)phthalocyanines in concentrated sulfuric acid solutions at different temperature values. The phenomenon of stepped protonation of the complexes was discovered, and spectra of separate protonated forms were obtained. The number of the protonated forms was different for various functional derivatives of copper(II)phthalocyanines. Quantitative characteristics of stability of the complexes were obtained, and the mechanism of transfer of the electron effects of the substituents onto molecule coordination center was studied. In the present work analogous investigation of the complexes of cations with different electron structure Cu
2+ and Al
3+ with composition CuPc(4-NO
2)
4 (
I), CuPc(4-Br)
4(5-NO
2)
4 (
II), (OH)AlPc(4-NO
2)
4 (
III) and (OH)AlPc(4-Cl)
4(5-NO
2)
4 (
IV) is carried out. Compounds were synthesized and identificated by O. Shishkina, V. Mayzlish and G. Shaposhnikov [
1]. By spectrophotometrical investigation and ZINDO1 calculations one protonated form is identificated for CuPc(4-Br)
4(5-NO
2)
4 and (OH)AlPc(4-NO
2)
4 and two protonated forms are identificated for CuPc(4-NO
2)
4 (the result is in good content with data of [
2]) and (OH)AlPc(4-Cl)
4(5-NO
2)
4. In the last case the acid-base titration is carried out, and values of stepped protonation constants are obtained. They characterize the complexes as weak bases in regard to H
2SO
4.
![Molecules 05 01461 i022]()
Total kinetic characteristic of dissociation of the metal - nitrogen bonds of the complexes is obtained. For Cu(II) phthalocyanines and (OH)AlPc(4-NO2)4 the dissociation rate is proportional to [MPc(R)n] and [H3O+]2. There is a weak extreme dependence of the dissociation rate from the solvent composition for CuPc(4-Br)4(5-NO2)4 due to change of the complex form in 17.0 M H2SO4. The electron influence of the substituents onto the reaction center is considered taking into account the complicated mechanism of activation of the reaction system and peculiarities of the molecular structure of the macrocyclic complexes.
References
- Shaposhnikov, G. P.; Kulinich, V. P.; Mayzlish, V. E. Advances in Porphyrin Chemistry; Golubchkov, O. A., Ed.; Volume V. 2, SPb; 1999; pp. 190–218. [Google Scholar]
- Negrimovsky, V. M.; Derkacheva, V. M.; Kalija, O. L.; Luk‘anetz, E. A. Zh. Obshch. Khim. 1991, 61, 460–470. (in Russian).
Thermochemical Study of Some Substituted Porphyrins
Dmitrii R. Zakirov,
Michael I. Bazanov,
Alexei V. Volkov
and
Alexander S. Semeikin
Department of Analytical Chemistry, Ivanovo State University of Chemistry and Technology, Ivanovo, Russia; e-mail:
The use of porphyrins and its complexes in medicine is of a great interest. The thermodynamic parameters of compounds such as combustion and formation enthalpies are the key values for the modeling of some biological processes and performing quantitative calculations. In the present paper the energy of combustion of tetraphenylporphin (TPhP) and etioporphyrine-1 (EP-1) was determined in the oxygen medium by the bomb calorimetry method. The structural formulas of the compounds under study are:
The CO2 content analysis of the reaction products by the Rossini technique was done for the evaluation of the completeness of combustion studied compounds. The standard enthalpies of combustion and formation of tetraphenylporphin (TPhP) and etioporphyrine-1 (EP-1) in the crystalline state (298 K) were calculated on the base of experimental data obtained.
The experimental magnitudes of combustion and formation enthalpies were compared with calculated ones by Hendrick technique. Good agreement of the calculated and experimental values was shown (
Table 1).
Table 1.
Experimental and calculation magnitudes of combustion and formation enthalpies
Table 1.
Experimental and calculation magnitudes of combustion and formation enthalpies
| Compound | –ΔcH0exp., кJ/mole | –ΔcH0calc., кJ/mole | δ,% | ΔfH0exp.,кJ/мole |
| TPhP | 22971.70±33.6 | 23112.50 | 0.61 | 1174.80 |
| EP-1 | 16676.58±42.47 | 16983.06 | 1.80 | -773.80 |
Ruthenium(IV)Tetraphenylporphine: Synthesis and Reactions in Proton Donating Solvents
Elena Yu. Tulaeva*,
Tatiana N. Lomova
and
Olga G. Balina
Institute of Solution Chemistry of Russian Academy of Sciences, Ivanovo, Russia
The complex of ruthenium(IV) with tetraphenylporphine in the form (PhO)2RuTPP has been prepared by the complex formation reaction of H2TPP with K2RuO4 in boiling phenol. The complex was separated by column chromatography on Al2O3 using CHCl3 as an eluent and identified by thin-layer chromatography and UV-Visible spectroscopy.
To determine the metalloporphyrin forms in proton donating solvents the dependence of their UV-Vis spectra on the solvent nature was investigated. It was established that in concentrated sulphuric acid (>17 M) complex (PhO)2RuTPP exists in molecular and does not form H-bonds with the solvent protons. (PhO)2RuTPP does not undergo complete dissociation of M-N bonds under heating in this medium.
In the mixed media HOAc - 1
.10
-3 ÷ 5 mol
.l
-1 H
2SO
4 the (PhO)
2RuTPP forms a π-cation radical [
1] with absorbtion maxima 582 and 645 nm according to equilibrium (1)
The rate of reaction (1) was measured in the HOAc - 1÷4 mol.l-1 H2SO4 at 351÷356К. kobs value increases with the increase of H2SO4 concentration and temperature. kobs vs. C0H2SO4 dependence discussed in the report is non-linear. Activation parameters of reaction (1) strongly depend on the H2SO4 concentration. This fact is the feature of π-cation radical formation as far as activation parameters of metaloporphyrins dissociation are almost independent from acid concentration.
Presence of π-donating bond Ru→N owing to d4 electron configuration of Ru(IV) is the reason of super-stability of (PhO)2RuТPP.
References:
- Carnieri, N.; Harriman, A. Inorg. Chim. Acta 1982, 62, 103.
Axial Ligand Substitution in Bis-Isocyanide Complexes of Ru(II)- Octaphenyltetraazaporphine
Sergei I. Vagin,
Pavel A. Stuzhin
and
U. Ziener
Ivanovo State University of Chemistry and Technology Ivanovo, Russia
The extracomplexes of ruthenium(II)octaphenyltetraazaporphine with different axial ligands (L)2RuOPTAP are very stable in general. For example, according to TGA data the coordinated pyridine in (Py)2RuOPTAP splits off at more than 200°C, when the porphyrazine macrocycle is no longer stable.
However, one molecule of isocyanide in bis-isocyanide complexes (R-NC)
2RuOPTAP (R=t-Bu, c-hex) appears to be quite labile due to the strong trans-effect of the opposite R-NC molecule. The investigation of interaction of (t-BuNC)
2RuOPTAP with pyridine has shown that only one molecule of t-BuNC is substituted by pyridine at the temperature lower 100°C with the formation of so-called mixed-ligand complex. In the studied range of pyridine concentrations in toluene the substitution reaction has the first order by ruthenium complex and zero order by pyridine, what agrees with dissociative mechanism of the process:
This mechanism is well known for reactions of (L)
2FeOPTAP, (L)
2FePc and (L)
2RuPc [
1,
2,
3,
4,
5]. In the great excess of pyridine the substitution rate is determined by dissociation of bisisocyanide complex (forward reaction with rate constant k
f). The kinetic data obtained for this process in toluene are presented in
Table 1.
Table 1.
| Concentration of pyridine, mol/l | Temperature,°C (К) | kf, c-1 | E act, kJ |
| 9.89 | 71.5 (344.7)
58.5 (331.7)
45.2 (318.4) | 0.0260±0.0003
0.00729±0.00006
0.00151±0.00001 | 98.5 |
| 4,94 | 71.5 (344.7)
58.5 (331.7)
45.2 (318.4) | 0.0282±0.0005
0.00713±0.00006
0.00158±0.00001 | 100.0 |
| 2.47 | 71.5 (344.7)
58.5 (331.7)
45.2 (318.4) | 0.0269±0.0004
0.00722±0.00005
0.00155±0.00001 | 98.9
Eact(average)= 99.1±3 |
The interaction of (R-NC)2RuOPTAP with different N-heterocycles in similar conditions leads to the formation of corresponding mixed-ligand complexes. The complexes (Py)(RNC)RuOPTAP and (1-CH3Im)(c-hexNC)RuOPTAP (1-CH3Im = 1-methylimidazole) as well as dimeric [(R-NC)RuOPTAP]2(μ-diPy) with dipyridyl bridging ligand (μ-diPy) were synthesized in quantitative yield. The structure of synthesized complexes was verified by UVVIS-, IR- and 1H-NMR-spectroscopy.
The IR-spectra of mixed-ligand complexes show the significant shift of -N≡C vibrations towards the low frequency in comparison with corresponding bis-isocyanide complexes (RNC)
2RuOPTAP.
1H-NMR-spectra of (L)(R-NC)RuOPTAP complexes contain signals of axial ligands protons which differ from the corresponding bis-adducts (L)
2RuOPTAP or (R-NC)
2RuOPTAP [
6].
References:
- Jones, J.G.; Twigg, N.V. J. Chem. Soc. Dalton Trans. 1978, 1709.
- Doeff, M.M.; Sweigart, D.A. Inorg. Chem. 1981, 20, 1683.
- Martinsen, J.; Miller, M.; Trojan, D.; Sweigart, D.A. Inorg. Chem. 1980, 19, 2162.
- Nyokong, T. J. Chem. Soc. Dalton Trans. 1983, 3601.
- Stynes, D.V. Inorg. Chem. 1977, 16, 1170.
- Stuzhin, P.A.; Vagin, S.I.; Hanack, M. Inorg. Chem. 1998, 37, 2655. [PubMed]
The Solvatation and the Complex Stability of Ag+ with 18-Crown-6 Polyether in H2O-EtOH Solvents.
Tatiana Usacheva*,
Valentin Sharnin
and
Sergei Ledenkov
Ivanovo State University of Chemistry and Thecnology, Ivanovo, Russia
The interest in studying reactions with crown-ethers is based upon the similarity of their properties with properties of natural biologic membranes. The complex stability in a solution depends on the nature and the composition of solvents. The formation of molecular or metal complexes is competitive with the solvatation of all participants of the reaction. It has been previously shown that the complex stability in binary solvents (H
2O-DMSO) can be of more value than in individual ones. The complex stability is the solvatation function of thermodynamic contributions of the reagents:
where: Δ
trG°(AgL
+), Δ
trG°(Ag
+), Δ
trG°(L) – are changes of Gibbs energy of the transfer of complex ion, metal and ligand from an individual to binary solvent.
The change of enthalpy of the reaction with the transfer from an individual solvent to a binary one takes place too:
where: Δ
trH°(Ag
+), Δ
trH°(L), Δ
trH°(AgL
+) – the transfer enthalpy of Ag
+, L and AgL
+ from an individual solvent to a binary one.
The effect of the composition of aqueous-ethanol solvents on complex stability of Ag+ with crown-ether 18-crown-6 has been investigated. Using the method based on the thermodynamic characteristics of solvatation of metal-ion, ligand and complex-ion the interpretation of the results has been given.
The complex formation of Ag
+ with 18-crown-6 and the solvatation of Ag
+ and 18-crown-6 have been studied using calorimetric and potentiometric methods in aqueous-ethanol solvents with various content of EtOH at 298.15K.
The solvatation of Ag+ in water is a more endothermic process than in ethanol. The maximum of ΔtrH°(Ag+) is found at 0.1mol.fraction of ethanol. The similar dependence of ΔtrH°(M+) H2O-EtOH =f (X EtOH ) was found previously for cations of alkali metals.
It was determined that the solvatation of 18-crown-6 is stronger in water than in ethanol. The solvatation of crown-ether in the solution depends upon the interaction between O-donor atoms of a macrocycle and H- atoms of solvent.
The complex AgL+ is more stable in ethanol than in water. With increasing the concentration of ethanol in the solution the enthalpy of complex formation decreases. The enthalpy contribution into the complex stability is a determining factor in all ethanol-water mixtures.
Hence it was established that the contribution of ΔtrH°(AgL+) destabilises the complex AgL+ with the reaction transfer (3) from water to water-ethanol solutions. The contribution of ΔtrH°(L) stabilises the complex ion. The contribution of the solvatation of Ag+ is not a determining factor in a reaction enthalpy increase at high concentrations of ethanol in the solvent. If the concentration of EtOH < 0.3 mol.fraction in the solvent, the solvatation of Ag+ will be of smaller value, this resulting in an increase of the reaction enthalpy and the complex stability AgL+.
Synthesis and Studies on Coordination Properties of meso-Phenyl Substituted Octamethyl Porphyrins.
Elena K. Vlasova,
Elizaveta M. Kuvshinova,
Alexander S. Semeikin
and
Oleg A. Golubchikov
Ivanovo State University of Chemistry and Technology, Ivanovo, Russia
5-Monophenyloctamethylporphyrin (I), 5,10-diphenyloctamethylporphyrin (II), 5,10,15-triphenyloctamethylporphyrin (III) and 5,10,15,20-tetraphenyloctamethylporphyrin (IV) were synthesized.
A spectral analysis of porphyrins (I-IV) and molecular mechanics calculations show that synthesized porphyrins are distorted and a degree of their deformations increases gradually in the order I<II<III<IV.
By kinetic studies of complex formation between (I-IV) and cupric and zinc acetates in pyridine it is found that the reaction rates increase from (I) to (IV). Apparently, it is caused by more effective solvation of nonsymmetrically substituted porphyrins (II, III) in a transient state than symmetrical (IV) that is exhibited in some magnification of reaction rates and magnitudes of activation energy and entropy.
The main reasons for the growth of a reactivity in the series of porphyrins I<II<III are the increasing in basicity of tertiary nitrogen atoms, forming coordination bonds with solvated metal cations in the transient state, according to increasing degree of macrocycle deformations in this series.
In acetic acid the opposite dependence in the number of porphyrins (I-IV) is observed. The diminution of reaction rates in acetic acid is influenced by porphyrin specific solvation, the role of which is increased in accordance with growing tetrapyrrolic aromatic nucleus deformations in the series of porphyrins (I-IV).