Secondary metabolites of marine origin are a source of new molecular architectures with interesting and promising biological activities.
For some time our group has been carrying out a general program of discovery and development of new compounds with antihelmintic activity. For this purpose we have chosen a group of natural prod-ucts isolated from sponges, which have displayed a very high antihelmintic activity. Such is the case of the Bengamides (1) and Micotiazol (2).
From a structural point of view these compounds share a common molecular pattern, in which a central heterocyclic ring, biogenetically derived from aninoacids, simultaneously bears sidechains with both lipo- and hydrophilic character. Molecular simplification of structures with proven biological ac-tivity is a classical tool used in Pharmaceutical Chemistry to obtain new lead compounds. This meth-odology was applied in our group starting from the basic structural patterns found in compounds of 1 and 2.
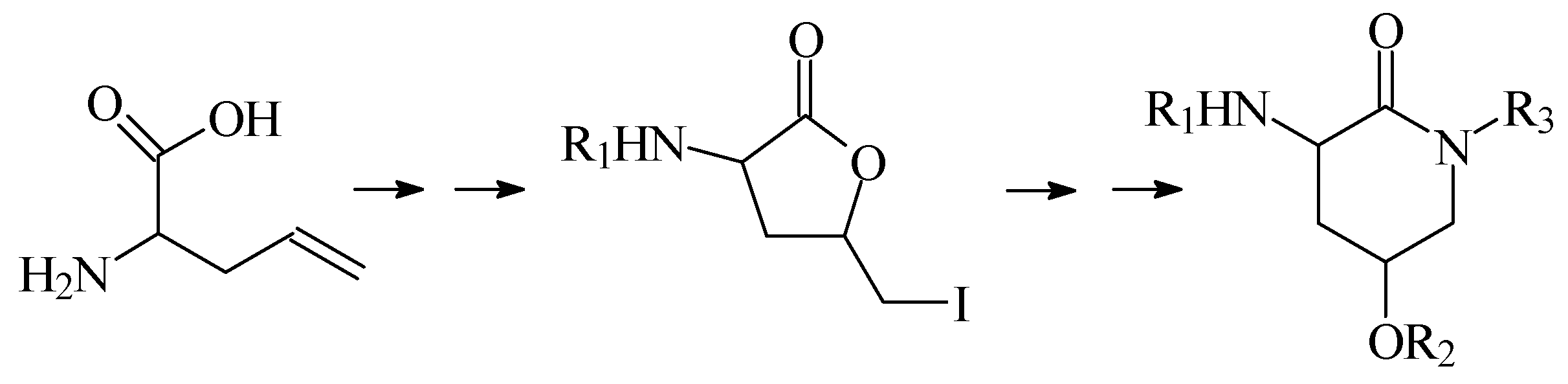
In this lecture we will first present our results when a group of derivatives 2-amino-4-hydroxy-δ-valerolactam were prepared via a synthetic sequence involving lactonization followed by a lactone-lactam exchange, as shown in
Scheme 1.
Second, we will describe the synthesis of 1,3-thiaza-2,4-disubstituted systems from acyclic precur-sors. We will discuss the results of different condensation and cyclodehydration methodologies, as well as the methods used to carry out controlled oxidations of the central heterocyclic system (
Scheme 2).
In all cases the antihelmintic activity of the synthesized compounds and the biological models used to evaluate these activities will be presented.