Syntheses of Furo[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines and Furo[2`,3`: 5,6]-pyrimido[3,4-b][2,3-e]indolo[1,2,4]triazine as a New Ring System
Abstract
:Introduction
Results and Discussion
Experimental
General
4,5-Di-(2-furyl)-2-(ethoxyethylideneamino)furan-3-carbonitrile (2)
5,6-Di-(2-furyl)-1H-3H-4-imino-2-methyl-3-phenylaminofuro[2,3-d]pyrimidine (3)
3-(p-Fluorobenzylamino)-5,6-di-(2-furyl)-3H-2-methylfuro[2,3-d]pyrimidine-4-imine (4)
5,6-Di-(2-furyl)-3H-2-methylfuro[2,3-d]pyrimidine-4-thione (5)
5,6-Di-(2-furyl)-1H-4H-furo[2,3-d][1,3-thiazine]-4-imino-2-thione (6)
5,6-Di-(2-furyl)-1H-3H-3-phenylfuro[2,3-d]pyrimidine-4-imine-2-one (7)
5,6-Di-(2-furyl)-2-(hydrazinoethyleneamino)furan-3-carbonitrile (8)
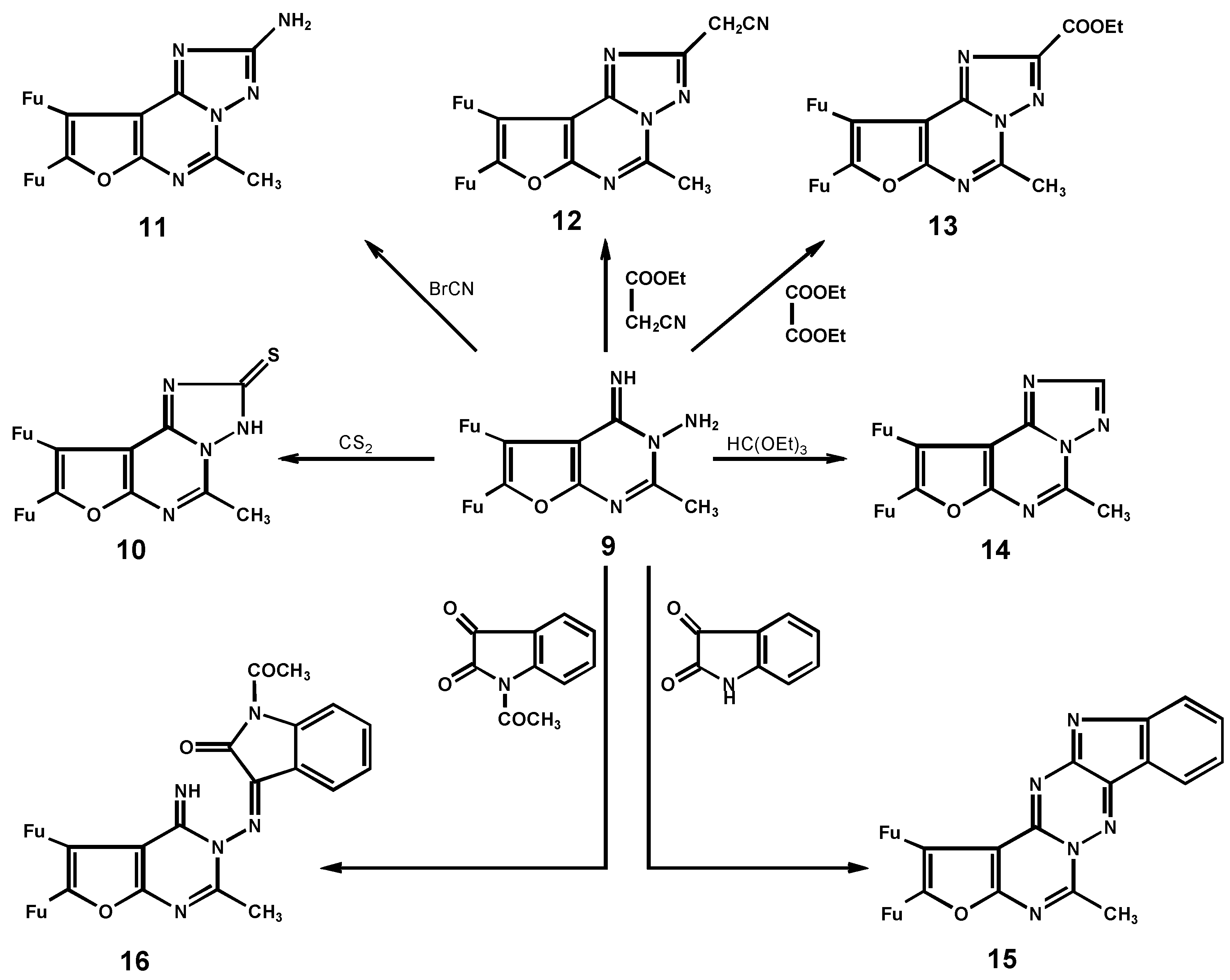
5,6-Di-(2-furyl)-3H-4H-4-imino-2-methylfuro[2,3-d]pyrimidine-3-amine (9)
8,9-Di-(2-furyl)-2,3-dihydro-5-methylfuro[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-2-thione (10)
8,9-Di-(2-furyl)-5-methylfuro[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-2-thione (11)
2-Cyanomethyl-8,9-di-(2-furyl)-5-methylfuro[3,2-e][1,2,4]triazolo[1,5-c] pyrimidine (12)
8,9-Di-(2-furyl)-2-ethyl-5-methylfuro[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-2-thione (13)
8,9-Di-(2-furyl)-3-methylfuro[2,3-e][1,2,4]triazolo[1,5-c]pyrimidine (14)
12,13-Di-(2-furyl)-9-methylfuro[2`,3`:5,6]pyrimido[3,4-b][2,3-e]indolo[1,2,4]triazine (15)
5,6-Di-(2-furyl)-3H-4H-4-imino-2-methylfuro[2,3-d]pyrimidine-1-(N-acetyl-2-oxo-isatin-3-yliden-amine (16)
| Comp. No. | m.p. oC | Mol. Formula (mol. wt) | Analysis (Calcd/Found) | |||
|---|---|---|---|---|---|---|
| C | H | N | S | |||
| 2 | 115-117 | C17H14N2O4 | 65.80 | 4.56 | 9.03 | |
| (310.3) | 65.77 | 4.51 | 8.99 | |||
| 3 | 179-181 | C21H16N4O3 | 67.73 | 4.34 | 15.05 | |
| (372.4) | 67.33 | 4.36 | 15.06 | |||
| 4 | 130-132 | C22H16FN3O3 | 67.85 | 4.15 | 10.79 | |
| (389.4) | 68.00 | 4.13 | 10.66 | |||
| 5 | 180-182 | C15H10N2O3S | 60.39 | 3.39 | 9.39 | 10.75 |
| (298.3) | 60.71 | 3.41 | 9.31 | 10.71 | ||
| 6 | 223-225 | C14H8N2O3S2 | 53.14 | 2.55 | 8.86 | 20.27 |
| (316.4) | 53.33 | 2.61 | 8.81 | 20.40 | ||
| 7 | 238-240 | C20H13N3O4 | 66.85 | 3.65 | 11.70 | |
| (359.3) | 66.79 | 3.51 | 11.40 | |||
| 8 | 150-152 | C15H12N4O3 | 60.80 | 4.09 | 18.91 | |
| (296.3) | 60.79 | 4.08 | 19.00 | |||
| 9 | 222-224 | C15H12N4O3 | 60.80 | 4.09 | 18.91 | |
| (296.3) | 60.81 | 4.08 | 19.01 | |||
| 10 | 275-277 | C16H10N4O3S | 56.78 | 2.98 | 16.56 | 9.48 |
| (338.4) | 56.56 | 2.96 | 16.61 | 9.41 | ||
| 11 | 230-232 | C16H11N5O3 | 59.81 | 3.46 | 21.80 | |
| (321.3) | 59.88 | 3.47 | 21.51 | |||
| 12 | 209-211 | C18H11N5O3 | 62.61 | 3.22 | 20.29 | |
| (345.3) | 62.81 | 3.41 | 20.41 | |||
| 13 | 189-191 | C19H14N4O5 | 60.30 | 3.74 | 14.81 | |
| (378.4) | 60.44 | 3.81 | 14.72 | |||
| 14 | 218-220 | C16H10N4O3 | 62.74 | 3.30 | 18.30 | |
| (306.3) | 62.75 | 3.32 | 18.47 | |||
| 15 | 295-300 | C23H13N5O3 | 67.80 | 3.22 | 17.19 | |
| (407.4) | 67.74 | 3.21 | 17.31 | |||
| 16 | 252-254 | C25H17N5O5 | 64.22 | 3.67 | 14.98 | |
| (467.5) | 64.00 | 3.91 | 14.86 | |||
| Comp. No. | IR cm-1 | 1H NMR (δ, ppm) |
|---|---|---|
| 2 | 3010 (CH aromatic); 2980, 2920 (CH-aliphatic); 2210 (CN); 1615 (C=N) | DMSO-d6: 1.60 (t, 3H, CH3); 2.65 (s, 3H, CH3); 4.35 (q,2H, CH2); 6.51-7.81 (m, 6H, furan protons) |
| 3 | 3450, 3398, 3304 (NH + NH2); 3040,3010 (CH-Ar); 1610 (C=N) | CDCl3: 2.95(s, 3H, CH3); 6.93 (brs, 1H, NH, exchangable); 7.05-7.85 (m, 11H, furan+ aromatic protons); 8.00 (s, br, 1H, NH, exchangable) |
| 4 | 3380 (NH); 3050 (CH-Ar); 1610 (C=N) | CDCl3: 2.89(s,3H,CH3); 4.68 (s, 2H, NCH2); 5.90 (brs, 1H,NH, exchangable); 6.31-7.55 (m, 10H, furan protons+ArH) |
| 5 | 3448 (NH); 3060 (CH-Ar); 1606 (C=N); 1246 (C=S) | CDCl3: 2.95 (s, 3H, CH3); 6.10-7.81(m, 7H, furan pro-tons+NH, exchangable) |
| 6 | 3430, 3318 (NH); 3038 (CH aromatic);1250 (C=S); 1608 (C=N); | DMSO-d6: 4.58 (brs, 1H, NH, exchangable); 6.51-7.85 (m, 6H, furan protons); 9.08 (brs, NH, exchangable) |
| 7 | 3380, 3290(NH); 3040 (CH aromatic);1680 (C=O); 1602 (C=N) | DMSO-d6: 5.81 (s, 1H, NH, exchangable); 6.71-7.85 (m, 11H, furan protons+ArH); 12.21 (br, 1H, NH-amide). |
| 8 | 3454, 3300, 3290 (NH+ NH2); 2208(CN); 1612 (C=N) | |
| 9 | 3310, 3250 (NH+ NH2); 3040 (CH-Ar);1615 (C=N) | DMSO-d6: 2.67(s, 3H, CH3); 4.93(brs, 2H, NH2, exchange-able); 6.45(brs, 1H, NH, exchangable); 6.73-7.85 (m, 6H, furan protons) |
| 10 | 3350(NH); 3085 (CH-Ar); 1612(C=N); 1250 (C=S). | DMSO-d6: 2.85(s, 3H, CH3); 3.75 (s, 1H, NH, exchange-able); 6.61-7.80 (m, 6H, furan protons) |
| 11 | 3420, 3380 (NH2); 3040 (CH-Ar); 1610 (C=N) | DMSO-d6: 2.66(s, 3H, CH3); 5.81 (s, 2H, NH2, exchange-able); 6.41-7.60 (m, 6H, furan protons) |
| 12 | 3050 (CH-Ar); 2220 (CN); 1616 (C=N) | CDCl3: 2.96(s, 3H, CH3); 4.65 (s, 2H, CH2); 6.31-7.42 (m,6H, furan protons) |
| 13 | 3040(CH-Ar); 2950, 2860 (CH-aliphatic); 1735(CO-ester); 1612 (C=N). | CDCl3: 2.65(s, 3H, CH3); 1.50 (t, 3H, CH3); 4.44-4.65 (q,2H, OCH2); 6.55(dd, 2H, furan 4-H); 6.90 (t, 2H, furan 3-H); 7.55 (d, 2H, furan 2-H) |
| 14 | 3060 (CH-Ar); 1600 (C=N) | DMSO-d6: 2.84(s, 3H, CH3); 6.44-7.51 (m, 6H, furan pro-tons); 8.31 (s, 1H, CH-triazole) |
| 15 | 3040 (CH-Ar); 1608 (C=N) | DMSO-d6: 2.72(s, 3H, CH3); 6.83 -7.91 (m,10H, furan pro-tons + NH, exchangable) |
| 16 | 3250 (NH); 3050 (CH-Ar); 2960 (CH-aliphatic); 1683 (C=O); 1585 (C=N); | DMSO-d6: 2.80(s, 3H, CH3); 2.92(s,3H, CH3); 6.41-7.83(m,11H, furan protons + NH,exchangable) |
References and Notes
- Pemmsin, M.; Lnu-Due, C.; Hoguet, F.; Gaultier, C.; Narcisse, J. Eur. J. Chem. 1988, 23, 543.
- Cannito, A.; Pemmsin, M.; Lnu-Due, C.; Hoguet, F.; Gaultier, C.; Narcisse, J. Eur. J. Chem. 1990, 25, 635.
- Smith, P.A.S.; Kan, R.O. J. Org. Chem. 1964, 29, 2261.
- Nega, S.; Aionso, J.; Diazj, A.; Junquere, F. J. Heterocycl. Chem. 1990, 27, 269.
- Tetsuo, S.; Mikio, T.; Hidetoshi, H.; Daijiro, H.; Akira, I. Jpn. Kokai Tokyo Koho JP 1987, 62, 132, 884 (C. A. 1987, 107: 198350h).
- Chakaravorty, P.K.; Grelnlee, W.J.; Dooseap, K.; Mantlo, N.B.; Patchett, A.A. A.P.C.T. Int. Appl. WO 92.20.687.156, 1992. (C.A. 1993, 118: 213104d).
- Shishoo, C.J.; Jain, K.S. J. Heterocycl. Chem. 1992, 29, 883.
- Shimamure, H.; Terajima, K.; Kawase, A.; Ishizuka, Y.; Kimura, I.; Kamya, A.; Kataoka, M.; Sato, M. Jpn. Kokai Tokyo Koho Jp. 05, 112, 559 (C. A. 1993, 119: 160315k).
- Yamamoto, Y.; Seko, T.; Nakamura, H.; Nemoto, H.; Hojo, H.; Mukai, N.; Hashimoto, Y. J. Chem. Soc. Chem. Commun. 1992, 157.
- Edie, R.G.; Hackler, R.E.; Krumkains, E.V. Eur. Pat. Appl. Ep. 49 (C.A.: 1992, 116: 128957y).
- Gangjee, A.; Devraj, R.; Barrews, L.R. J. Med. Chem. 1994, 37, 1169. [PubMed]
- Gewald, K. Chem. Ber. 1966, 99, 1002.
- Fathy, N.M.; Hassan, N.A.; Heba, S.H.; Abdel-Megeid, F.M.E. J.Fac.Sci. Menoufeia Uni. 1996, 10, 205.
- Samples Availability: Not available.

© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Hassan, N.A. Syntheses of Furo[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines and Furo[2`,3`: 5,6]-pyrimido[3,4-b][2,3-e]indolo[1,2,4]triazine as a New Ring System. Molecules 2000, 5, 826-834. https://doi.org/10.3390/50600826
Hassan NA. Syntheses of Furo[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines and Furo[2`,3`: 5,6]-pyrimido[3,4-b][2,3-e]indolo[1,2,4]triazine as a New Ring System. Molecules. 2000; 5(6):826-834. https://doi.org/10.3390/50600826
Chicago/Turabian StyleHassan, Nasser A. 2000. "Syntheses of Furo[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines and Furo[2`,3`: 5,6]-pyrimido[3,4-b][2,3-e]indolo[1,2,4]triazine as a New Ring System" Molecules 5, no. 6: 826-834. https://doi.org/10.3390/50600826




