Results and Discussion
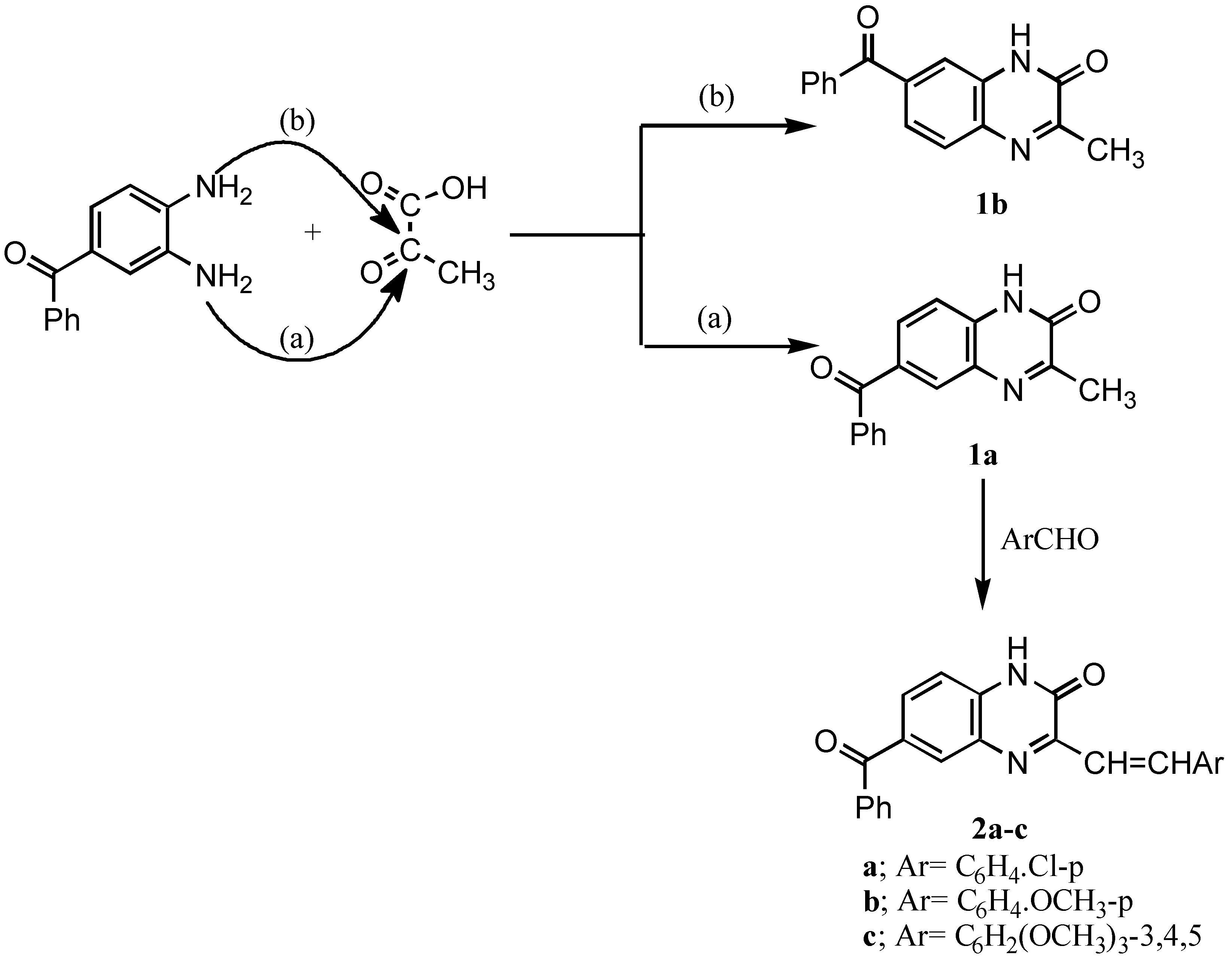
Condensation of 4-benzoyl-1,2-phenylenediamine with sodium pyruvate in acetic acid at room temperature furnished two products which showed analytical and spectral data in good agreement with the 3-methylquinoxalinone structures
1a,
b. The reaction proceeds due to the (–R) effect of the benzoyl group which deactivates the p-amino group, thus the m-amino group initiates the reaction to give 6-benzoyl-3-methyl-2(1H)quinoxalinone
1a as the main product (60%). The second isomer was obtained in 30% yield and identified as 7-benzoyl-3-methyl-2(1H)quinoxalinone
1b (
Scheme 1). The
1H NMR spectrum of
1a in DMSO-d
6 exhibited signals at 2.6 (3H, s, CH
3), 8.21 (1H, broad, NH; cancelled with D
2O). Also, the mass spectrum displayed a molecular ion peak at m/z 264 (70%). The proposed fragmentation pattern of
1a is illustrated in
Scheme 2.
6-Benzoyl-3-substituted styryl-2(1H)quinoxalinones 2a-c were obtained via fusion of 1a with aromatic aldehydes in the presence of piperidine at 160°C. The 1H NMR of 2b in DMSO-d6 showed signals at 4.2 (3H, s, OCH3), 7.20, 7.65 (2H, 2s, CH=CH). The coupling constant of the styryl protons is 14-15 Hz, indicating trans isomerism at the double bond.
It was reported that quinoxalinone was alkylated using dimethyl sulphate to furnish the corresponding N-methyl derivative [
10]. In a similar fashion compounds
1a,
b were alkylated with dimethyl sulphate in the presence of sodium hydroxide to afford the 6-and 7-benzoyl-1,3-dimethyl-2(1H)quinoxalinones
3a,
b, respectively (
Scheme 3). The
1H NMR spectrum of
3a in CDCl
3 showed signals at 2.6 (3H, s, CH
3), 3.6 (3H, s, N-CH
3). Also, when compounds
1a,
b were alkylated with ethyl chloroacetate in acetone using potassium carbonate as catalyst, the ester derivatives
4a,
b were produced. The
1H NMR spectrum of
4a in CDCl
3 showed 1.4 (3H, t, CH
3-ester), 2.4 (3H, s, CH
3), 4.2 (2H, q, OCH
2–ester), 4.9 (2H, s, N-CH
2).
Hydrazinolysis of 4a with hydrazine hydrate afforded 1-[6-benzoyl-3-methyl-2(1H)-quinoxalinone]acetic acid hydrazide (5). Its mass spectrum showed a molecular ion peak at m/z 336 (M+, 1.6%) and 321[M+-15 (CH3.)]. Refluxing of the hydrazide derivative 5 with aromatic aldehydes in ethanol furnished the corresponding 1-[6-benzoyl-3-methyl-2(1H)quinoxalinone]acetic hydrazones 6a,b. The 1H NMR spectrum of 6b showed signals 2.6 (3H, s, CH3), 4.0 (3H, s, OCH3) and 9.0 (1H, s, N=CH).
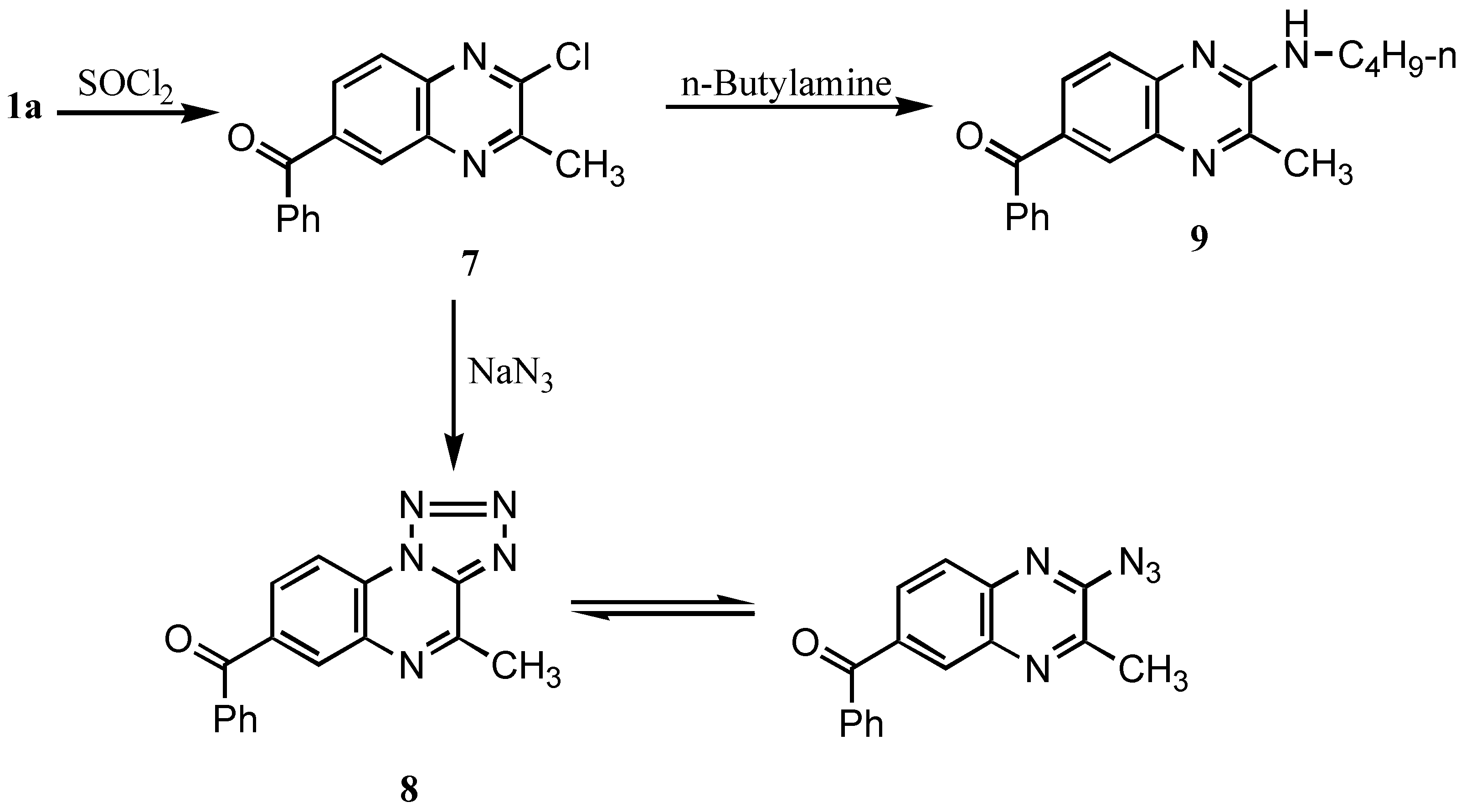
In addition, chlorination of
1a with thionyl chloride furnished 6-benzoyl-2-chloro-3-methylquinoxaline (
7) (
Scheme 4).
Its IR spectrum revealed the disappearance of the NH band, present in the parent compound. Also, its mass spectrum showed m/z 267 [M
+-15 (CH
3), 56.1%]. When the chloro derivative
7 was reacted with sodium azide in ethanol, the 7-benzoyl-4-methyltetrazolo[1,5-a]quinoxaline (
8) was obtained which was found to be in tautomeric equlibrium with the 2-azido derivative [
11]. Its IR spectrum displayed absorption bands at 1700 (C=O) and the characteristic absorption band for the azido group at 2240 cm
-1 [
12]. Also, its mass spectrum displayed a molecular ion peak m/z at 289 (M
+, 1.6%). Finally, condensation of
7 with n-butylamine in DMF furnished 6-benzoyl-2-butylamino-3-methylquinoxaline (
9). Its IR spectrum showed a NH band at 3250 cm
-1.
Anitmicrobial activities
The antimicrobial activities of the synthesized compounds
1a,
b,
2b,
4a,
b,
5 and
6b were determined by the agar diffusion technique [
13]. The organisms tested were
Staphyloccus aureus (NCTC 7447),
Bacillus cereus (ATCC-14579),
Serratia marcescens (IMRU 70),
Proteus merabitis (NCTC-289),
Aspergillus ochraceus Wilhelm (AUCC-230) and
Penicillium chrysogenum Thom (AUCC-530). The agar media were inoculated with test organisms and a solution of the tested compound in DMSO (250 μg/ml) was placed separately in cups (8 mm diameter) in the agar medium. Streptomycin (25 μg) and mycostatin (30 μg) were used as a reference for the antibacterial and antifungal activities, respectively. The inhibition zones were measured after 24 hr. incubation. The results of the antimicrobial activity tests are summarized in
Table 1.
Most of the synthesized compounds were found to possess varied antibacterial activities towards all the microorganisms used with minimal inhibitory concentration (MIC). However, none of the tested compounds showed superior activity than the reference drugs.
Experimental
General
Melting points were determined by an Electrothermal melting point apparatus and are uncorrected. Elemental analyses were carried out by Microanalytical Unit, Faculty of Science, Cairo University. IR spectra were recorded on a Shimadzu 440 spectrophotometer using KBr disks. 1H NMR spectra were measured on a Varian EM-360 90 MHz spectrophotometer (Varian, UK) using TMS as internal standard. The mass spectra were run by a Shimadzu-GC-MS-GP 100 EX using the direct inlet system.
3-Methyl-2(1H)quinoxalinones (1a,b)
A solution of 4-benzoyl-1,2-phenylenediamine (0.01mol) in acetic acid (50 mL) was treated with a solution of sodium pyruvate (0.01mol) in a minimum amount of water. The reaction mixture was stirred at room temperature for 2 hr and the solid thus obtained was subjected to fractional crystallization to give products
1a,
b (
Table 2). Spectral data:
1a: IR (cm
-1): 3320 (NH), 2950 (CH-aliphatic), 1690 (C=O);
1H NMR (ppm, DMSO-d
6): of
1a; 2.47 (3H, s, CH
3), 6.72-7.82 (8H, m, Ar-H), 8.43 (1H, broad, NH; exchangeable with D
2O). Mass spectrum (m/z): 264 (M, 70%), 236 (5.6%), 235 (6.8%), 195 (1.4%), 187 (19.6%), 159 (100%), 131 (23.5%), 118 (3.4%), 116 (1.9%), 105 (37.3%) and 77 (41.4%).
1b: 1H NMR (ppm, DMSO-d
6): 2.6 (3H, s, CH
3), 8.21 (1H, broad, NH), 6.8-7.4 (8H, m, Ar-H).
6-Benzoyl-3-substituted styryl-2(1H)quinoxalines (2a-c)
A mixture of (
1a, 0.01mol), the requisite aldehyde (0.01mol) and piperidine (0.5mL) was fused at 160°C for 2 hr. The obtained product was crystallized from the appropriate solvent (
Table 2) to afford compounds
2a-
c.
6-Benzoyl-3-(4-chlorostyryl)-2(1H)quinoxaline (2a)
IR (cm-1): 3340 (NH), 1680, 1650 (C=O). 1H NMR spectrum of 2a in DMSO-d6 exhibited signals at 7.16. 7.78 (2H, 2s, CH=CH), 7.89-8.20 (12H, m, Ar-H), 8.72 (1H, broad, NH; exchangeable with D2O).
6-Benzoyl-3-(4-methoxystryryl)-2-(1H)quinoxaline (2b)
IR (cm-1): 3080 (NH), 1682, 1645 (C=O). 1H NMR spectrum of 2b in DMSO-d6 exhibited signals at 4.2 (3H, s, OCH3), 7.20, 7.65 (2H, 2s, CH=CH), 7.90-8.4 (12H, m, Ar-H), 8.6 (1H, s, NH; exchangeable with D2O).
6-Benzoyl-3-(3,4,5-trimethoxystryryl)-2-(1H)quinoxaline (2c)
IR (cm-1): 3320 (NH), 1690, 1635 (C=O). 1H NMR spectrum of 2c in DMSO-d6 exhibited signals at 3.91 (6H, s, 2OCH3), 4.1 (3H, s, OCH3), 7.30, 7.42 (2H, 2s, CH=CH), 7.82-8.32 (10H, m, Ar-H), 9.21 (1H, s, NH; exchangeable with D2O).
6-Benzoyl or 7-benzoyl-1,3-dimethyl-2(1H)quinoxalines (3a,b)
Dimethyl sulphate (0.022 mol) was added to a solution of (
1a or
1b; 0.01 mol) in sodium hydroxide solution (10%, 50 mL). The reaction mixture was stirred at 60°C for 3 hr and the obtained solid was recrystallized to give (
3a,
b;
Table 2). IR spectrum of
3a revealed bands at 2980 (CH-aliphatic) and 1695, 1644 (CO), also
1H NMR spectrum of
3a in CDCl
3 showed signals at 2.6 (3H, s, CH
3), 3.6 (3H, s, N-CH
3), 7.9-8.5 (8H, m, Ar-H). IR (cm
-1) spectrum of
3b: 2950 (CH-aliphatic), 1693, 1638 (C=O).
1H NMR spectrum of
3b in CDCl
3 showed signals at 2.46 (3H, s, CH
3), 3.71 (3H, s, N-CH
3), 7.82-8.31 (8H, m, Ar-H).
Ethyl [6-or 7-benzoyl-3-methyl-2(1H)quinoxalinon-1-yl]acetate (4a,b)
A mixture of (
1a or
1b; 0.01 mol), ethyl chloroacetate (0.01 mol) and potassium carbonate (2 g) in acetone (50 ml) was refluxed for 6 hr. The obtained product was crystallized from the proper solvent to furnish (
4a,
b;
Table 2). IR spectrum of
4a: 2982 (CH-aliphatic), 1735, 1680 (C=O).
13C NMR of
4a DMSO-d
6 showed 15.3 (CH
3-ester), 21.2 (CH
3), 45.4 (N-CH
2), 58.3 (OCH
2-ester), 119-148.6 (13 C sp
2 carbon atoms) and 168.2, 170.4, 175.8 (3 C=O groups). IR spectrum of
4b afforded bands at 2985 (CH-aliphatic) and 1730, 1695 (C=O).
1H NMR spectrum of
4b in CDCl
3 showed signals at 1.2 (3H, t, CH
3-ester), 2.4 (3H, s, CH
3), 4.2 (2H, q, OCH
2-ester), 4.9 (2H, s, N-CH
2) and 7.1-8.0(8H, m, Ar-H).
[6-Benzoyl-3-methyl-2-quinoxalinon-1-yl]acetic acid hydrazide (5)
To a solution of (
4a, 0.01mol) in ethanol (50 ml), hydrazine hydrate (0.012 mol) was added. The reaction mixture was refluxed for 10 hr and the obtained product was crystallized from the suitable solvent to yield (
5;
Table 2). Its IR spectrum showed bands at 3300, 3200 (NH, NH
2), 1690, 1675 (C=O). Also its mass spectrum showed m/z 336 (1.6%), 321 (15.8%), 305 (84.8%), 277 (5.6%), 264 (5.0%), 249 (100%), 235 (3.1%), 216 (4.5%), 188 (2.2%), 130 (2.3%), 116 (2.3%), 105 (52.4%) and 77 (53.1%).
[6-Benzoyl-3-methyl-2(1H)quinoxalinon-1-yl]acetic acid hydrazones (6a,b)
A solution of the hydrazide derivative
5 (0.01mol) in ethanol (30 mL) was treated with benzaldehyde or p-methoxybenzaldehyde (0.01 mol). The reaction mixture was refluxed for 4 hr and the product that obtained after filtration was crystallized from the proper solvent to afford (
6a,
b;
Table 2). IR spectrum of
6a showed bands at 2990 (CH-aliphatic) and 1690, 1670 (C=O).
1H NMR spectrum of
6a in CDCl
3 exhibited signals at 2.45 (3H, s, CH
3), 4.83 (2H, s, N-CH
2), 7.40-8.22 (13H, m, Ar-H), 8.62 (1H, broad, NH; exchangeable with D
2O) and 9.10 (1H, s, N=CH).
1H NMR spectrum of
6b in CDCl
3 exhibited signals at 2.6 (3H, s, CH
3), 4.0 (3H, s, OCH
3), 4.8 (2H, s, N-CH
2), 7.2-8.1 (12H, m, Ar-H), 8.5 (1H, broad, NH) and 9.0 (1H, s, N=CH).
6-Benzoyl-2-chloro-3-methylquinoxaline (7)
To a solution of
1a, 0.01mol) in dioxane (20 mL) and DMF (5 mL), thionyl chloride (10 mL) was added. The reaction mixture was refluxed for 4 hr and the obtained solid was crystallized from the proper solvent to afford compound
7 (
Table 2). Its mass spectrum showed m/z at 267 [M
+-15 (CH
3); 56.1%], 254 (8.1%), 242 (14.3%), 240 (10.4%), 225 (8.8%), 184 (2.6%), 162 (100%), 105 (COPh; 3.6%) and 77 (73.2).
7-Benzoyl-4-methyltetrazolo[1,5-a]quinoxaline (8)
To a solution of
7 (0.01mol) in ethanol (50 mL), sodium azide (0.01 mol) was added and the reaction mixture was refluxed for 6 hr. The product obtained after filtration was crystallized from a suitable solvent to furnish
8 (
Table 2). Its IR spectrum exhibited the following bands: 2943 (CH-aliphatic), 2240 (N
3), 1700 (C=O), 1609 (N=N), 1586 (C=N).
1H NMR spectrum of
8 in CDCl
3 exhibited signals at 2.57 (3H, s, CH
3), 7.67-8.21 (8H, m, Ar-H). Also, its mass spectrum showed a molecular ion peak at m/z 289 (M
+, 1.6%), 235 (1.2%), 185 (12.4%), 139 (1.5%), 105 (COPh, 100%) and 77 (Ph, 77.2%).
6-Benzoyl-2-(n-butylamino)-3-methylquinoxaline (9)
A mixture of
7 (0.01mol) and n-butylamine (0.01 mol) in DMF (20 mL) was heated under reflux for 3 hr. After allowing the reaction mixture to cool to room temperature, it was poured into cooled water (100mL). The insoluble material was filtered off and crystallized from the appropriate solvent to give
9 (
Table 1). Its IR spectrum revealed bands at 3250 (NH), 2995 (CH-aliphatic) and 1688 (C=O). Mass spectrum of
9 exhibited a molecular ion peak at m/z 319 (25%) together with base peak at m/z 105 (100%).