A New Synthetic Route to Dihydrobenzopyran Via Tandem Demethylation Cyclisation
Abstract
:Introduction
Results and Discussion

| S.No | Reagents | Time(h) | Productc | Yield(%) |
| 1. | BF3.OEt2/ EtSH | 48a | - | - |
| 2. | AlCl3/EtSH | 12b | 2 3 | 40 20 |
| 3. | AlCl3/EtSH | 24b | 3 | 76 |
| 4. | HBr/AcOH | 12 | 3 | 40 |
| 5. | AlCl3/DMS | 24b | 4 | 62 |
| 6. | ZnCl2/EtSH | 48a | 4 | 24 |
| 7. | TiCl3/EtSH | 48a | - | - |
| 8. | TiCl4/EtSH | 48a | - | - |
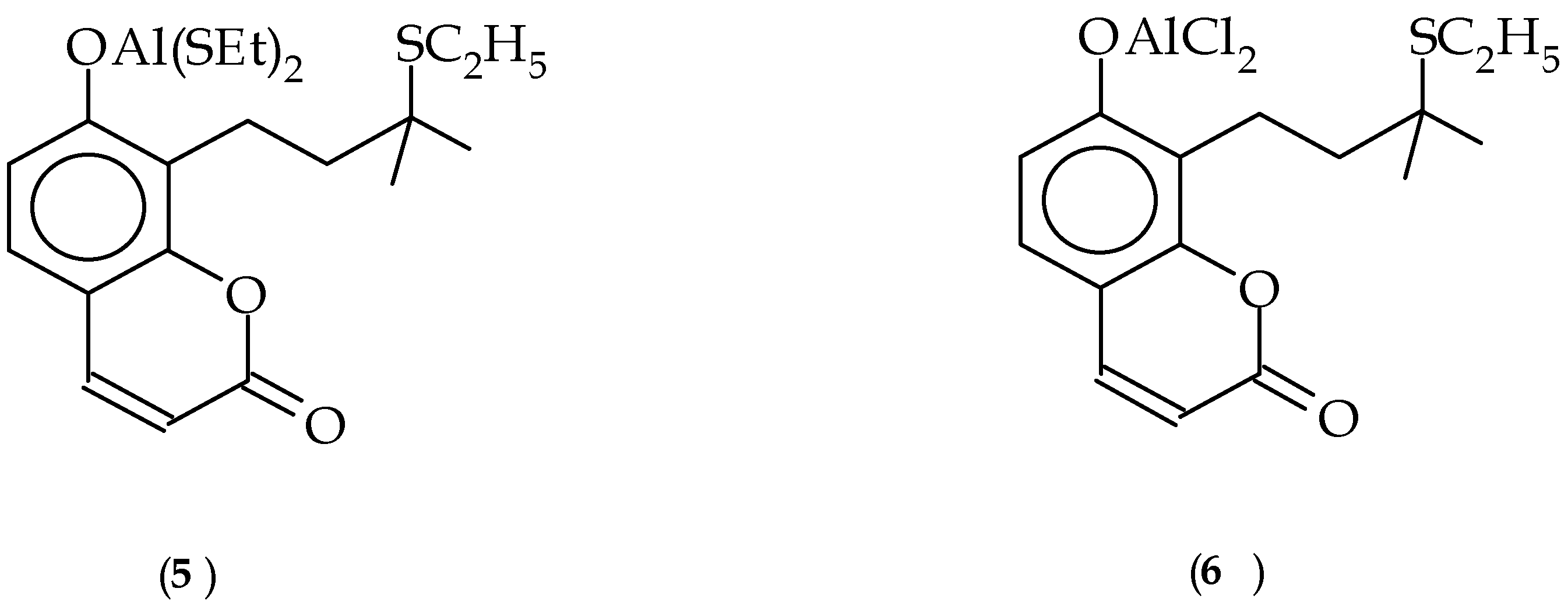
Experimental
General
7-Hydroxy-8-(3-methyl-3-thioethylbutyl)-coumarin (2)
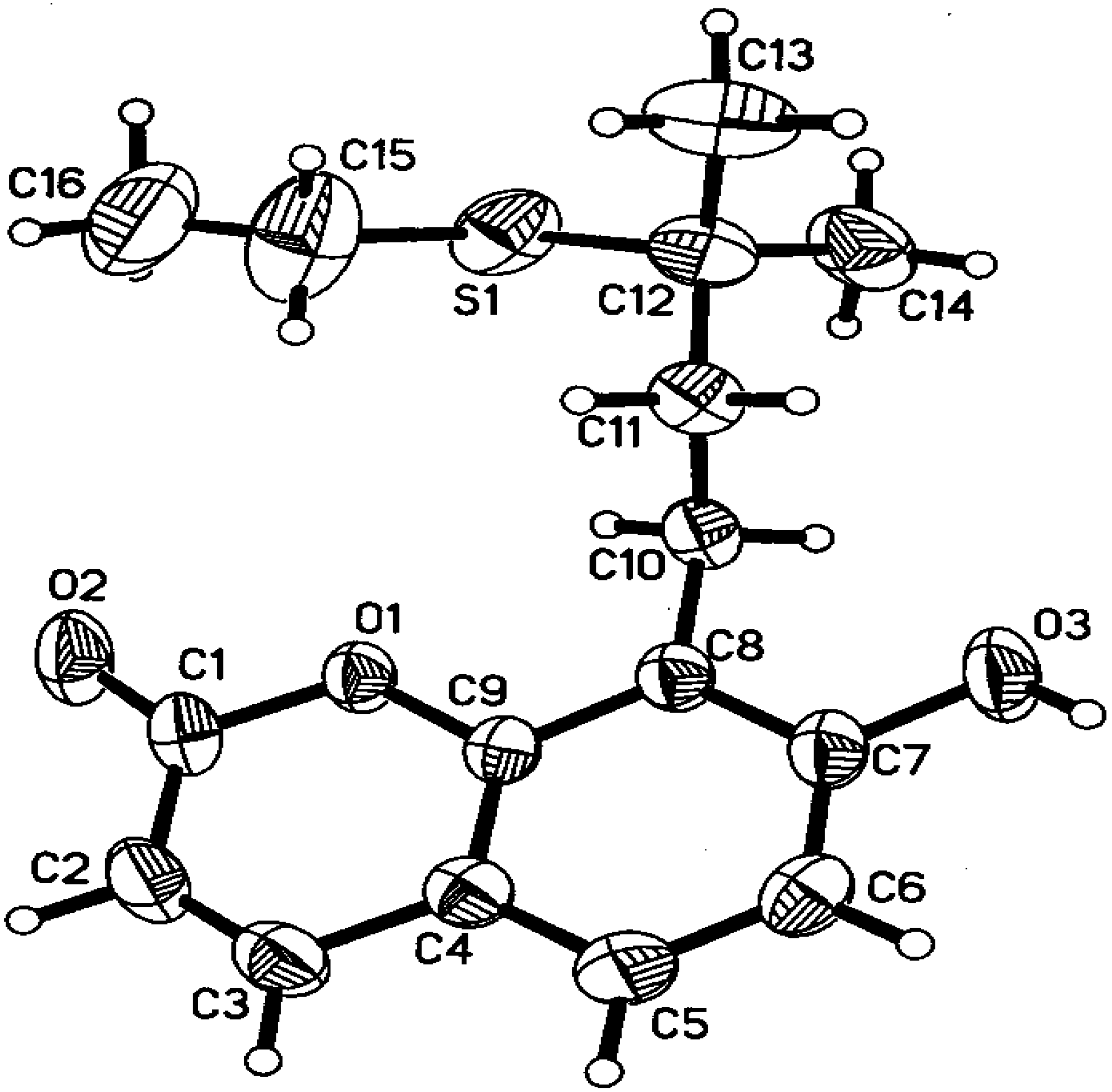
Crystal data
Structure solution and refinement
7,8-(11,11-Dimethyl pyrano)coumarin (3)
Osthenol (4)
Acknowledgements
References and Notes
- CAS-[484-12-8]
- Liu, J.H.; Zschocke, S.; Reininger, E.; Bauer, R. Inhibitory effects of Angelica Pubescens f. biser- rata on 5-Lipoxygenase and Cycloxygenase. Planta Medica 1998, 64, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, L.; Casiraghi, G.; Casnati, G.; Sartori, G. Selectivity in reactions between metal phenoxides and isoprene: Facile synthesis of 2,2-dimethyl chromans. Angew. Chem. Int. Ed. Engl 1978, 17, 684–686. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Kalyanaraman, P.S.; Muthukumaraswamy, N.; Pai, B.R. Xanthones of Gar-cinia Mangostana linn. Tetrahedron 1971, 3919–3926. [Google Scholar] [CrossRef]
- Miller, J.A.; Wood, H.C.S. Phosphate esters. Part I. The synthesis of phenolic isoprenoids from Allylic phosphates. J. Chem. Soc.(C) 1968, 1837–1843. [Google Scholar] [CrossRef]
- Bernard, A.M.; Cocco, M.T.; Onnis, V.; Piras, P.P. Facile Synthesis of 2,2-dimethyl chromans by Mo(CO)6. Synthesis 1998, 256–258. [Google Scholar] [CrossRef]
- Biggi, F.; Carloni, S.; Maggi, R.; Muchetti, C.; Rastelli, M.; Sartori, G. Reaction between phenols and isoprene under zeolite catalysis - Highly selective synthesis of chromans and o-isopentenyl- phenols. Synthesis 1998, 301–304. [Google Scholar] [CrossRef]
- Nilson, J.L.G.; Sievertsson, H.; Selander, H. Synthesis of methyl substituted 6-hydroxychromans, model compounds of tocopherols. Acta. Chem. Scand. 1968, 22, 3160–3170. [Google Scholar] [CrossRef]
- Richards, R.W.; Ronneberg, H. Synthesis of (-)-Δ9-6a,10a-trans tetrahydrocannabinol - BF3 cata-lyzed arylation by a homo cuprate. J. Org. Chem. 1984, 49, 572–573. [Google Scholar] [CrossRef]
- Node, M.; Nishide, K.; Fuji, K.; Fujita, E. Hard acid and soft nucleophile system. 2. Demethylation of methyl ethers of alcohol and phenol with an aluminium halide-thiol system. J. Org. Chem. 1980, 45, 4275–4277. [Google Scholar] [CrossRef]
- CAS [484-14-0]
- Sheldrick, G.M. SHELXS-97. University of Gottingen: Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97. University of Gottingen: Germany, 1997. [Google Scholar]
- Samples Availability: Available from MDPI.
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gopalakrishnan, G.; Kasinath, V.; Pradeep Singh, N.D.; Thirumurugan, R.; Sundara Raj, S.S.; Shanmugam, G. A New Synthetic Route to Dihydrobenzopyran Via Tandem Demethylation Cyclisation. Molecules 2000, 5, 880-885. https://doi.org/10.3390/50600880
Gopalakrishnan G, Kasinath V, Pradeep Singh ND, Thirumurugan R, Sundara Raj SS, Shanmugam G. A New Synthetic Route to Dihydrobenzopyran Via Tandem Demethylation Cyclisation. Molecules. 2000; 5(6):880-885. https://doi.org/10.3390/50600880
Chicago/Turabian StyleGopalakrishnan, Geetha, Viswanathan Kasinath, N. D. Pradeep Singh, R. Thirumurugan, S. Shanmuga Sundara Raj, and G. Shanmugam. 2000. "A New Synthetic Route to Dihydrobenzopyran Via Tandem Demethylation Cyclisation" Molecules 5, no. 6: 880-885. https://doi.org/10.3390/50600880




