Synthesis of Potassium (1,1-Dioxothiolan-3-yl)-dithiocarbamate
Abstract
:Introduction
Results and Discussion
Conclusions
Experimental
General
3-Aminothiolane
Potassium (1,1-dioxothiolan-3-yl)-dithiocarbamate
References and Notes
- Parkhomenko, P.I.; Vasiliev, A.N. SCK - a new low toxic fungicide from the family of dithiocarbamates. In Abstr. 9th Int. Congress of Pesticide Chemistry, London, 1998. Abstr. 1B-012.
- Register of pesticides and agrochemicals permitted for use in Ukraine. Univest Marketing: Kiev, 1999; p. 75.
- Castro, E.; Cortes, K.; Santos, J. Kinetics and mechanism of the reaction of carbon disulfide with piperidine in ethanol. J. Org. Chem. 1982, 47, 3774–3777. [Google Scholar] [CrossRef]
- Polackov, A.D.; Vasiliev, A.N. Optimization of the synthesis of potassium (1,1-dioxothiolan-3-yl) dithiocarbamate. Rus. Journ. Appl. Chem. 1995, 68, 1666–1668. [Google Scholar]
- Shkaraputa, L.N.; Kononov, A.V.; Polackov, A.D. Preparation of dithiocarbamates. Ukr. Chem. J. 1991, 9, 979–989. [Google Scholar]
- Polackov, A.D. Modelling and optimization of potassium (1,1-dioxothiolan-3-yl)-dithiocarbamate synthesis. Ph.D. Thesis, Institute of Bioorganic Chemistry and Petrochemistry, Kiev, 1992. [Google Scholar]
- Polackov, A.D.; Vasiliev, A.N. Investigation of base catalysis in dithiocarbamate formation. In Abstr. 35th Congr. IUPAC, Istanbul, 1995; Sec. 1-3. p. 624.
- Samples Availability: Available from MDPI.
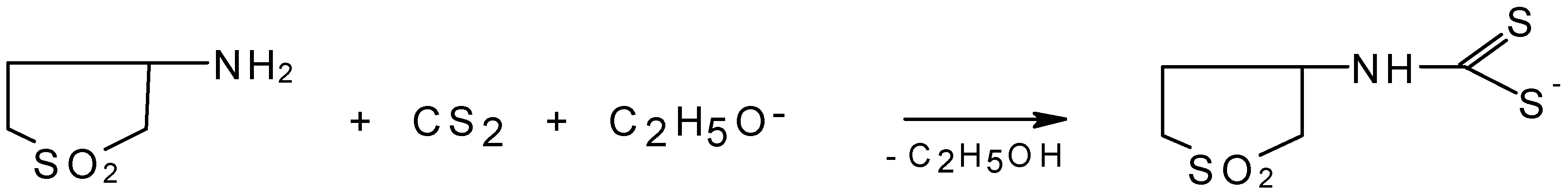
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Vasiliev, A.N.; Polackov, A.D. Synthesis of Potassium (1,1-Dioxothiolan-3-yl)-dithiocarbamate. Molecules 2000, 5, 1014-1017. https://doi.org/10.3390/50801014
Vasiliev AN, Polackov AD. Synthesis of Potassium (1,1-Dioxothiolan-3-yl)-dithiocarbamate. Molecules. 2000; 5(8):1014-1017. https://doi.org/10.3390/50801014
Chicago/Turabian StyleVasiliev, A. N., and A. D. Polackov. 2000. "Synthesis of Potassium (1,1-Dioxothiolan-3-yl)-dithiocarbamate" Molecules 5, no. 8: 1014-1017. https://doi.org/10.3390/50801014



