Lewis Acid Catalysis of the Diels–Alder Reaction Using Niobium and Tantalum Chlorides in the Presence of Coordinating Ligands
Abstract
:Introduction
Results and Discussion
Conclusions
Experimental
General
Synthetic procedures
References and Notes
- Kagan, H.B.; Riant, O. Chem. Rev. 1992, 92, 1007.
- Corey, E.J.; Imai, N.; Zhang, H.Y. J. Am. Chem. Soc. 1991, 133, 728. [CrossRef]
- Corey, E.J.; Ishimara, K. Tetrahedron Lett. 1992, 33, 6807.
- Evans, D.A.; Murry, J.A.; von Matt, P.; Norcross, R.D.; Miller, S.J. Angew. Chem. Int. Ed. Eng. 1995, 35, 798.
- Howarth, J.; Gillespie, K. Tetrahedron Lett. 1996, 37, 413.
- Bedekar, A.V.; Koroleva, E.B.; Andersson, P.G. J. Org. Chem. 1997, 62, 2518. [CrossRef]
- Hansen, K.B.; Leighton, J.L.; Jacobsen, E.N. J. Am. Chemical Soc. 1996, 118, 10924. [CrossRef]
- Shaus, S.E.; Branault, J.E.; Jacobsen, E.N. J. Org. Chem. 1998, 63, 403.
- Sigman, M.S.; Jacobsen, E.N. J. Am. Chem. Soc. 1998, 120, 5315. [CrossRef]
- Nesper, R.; Pregosin, P.S.; Puentener, K.; Woerle, M. Helv. Chim. Acta 1993, 76, 2239. [CrossRef]
- Narasaka, K.; Iwasawa, N.; Inoue, M.; Yamada, T.; Nakashima, M.; Sugimori, J. J. Am. Chem. Soc. 1989, 111, 5340. [CrossRef]
- Martinez, L.E.; Leighton, J.L.; Carsten, D.H.; Jacobsen, E.N. J. Am. Chem. Soc. 1995, 117, 5897. Larrow, J.F.; Schaus, S.E.; Jacobsen, E.N. J. Am. Chem. Soc. 1996, 118, 7420. Schaus, S.E.; Larrow, J.F.; Jacobsen, E.N. J. Org. Chem. 1997, 62, 4197.
- Nishiyama, H.; Kondo, M.; Nakamura, T.; Itoh, K. Organometallics 1991, 10, 500.
- Narasaka, K. Synthesis 1991, 1.
- Sample Availability: Not available.
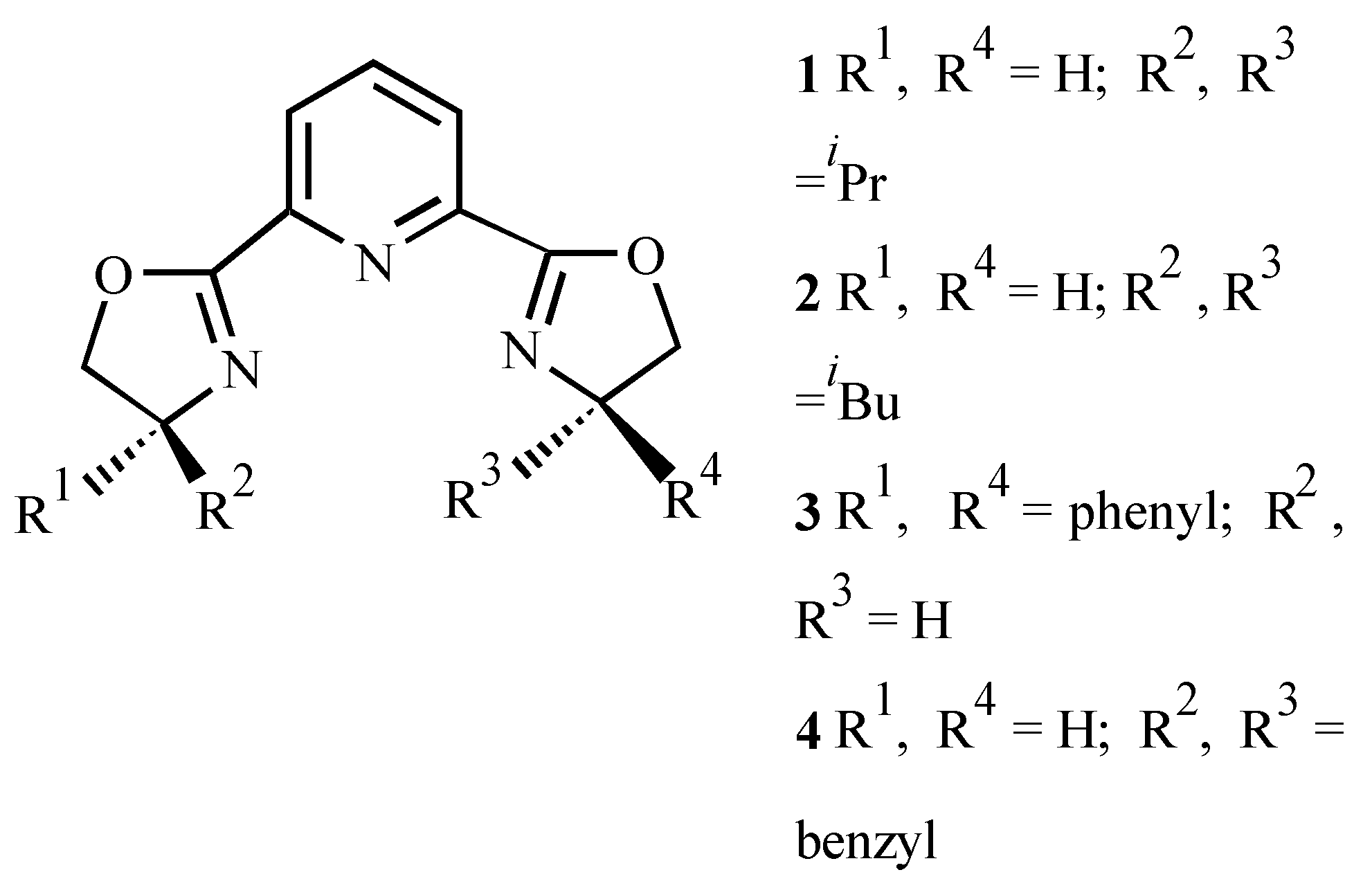
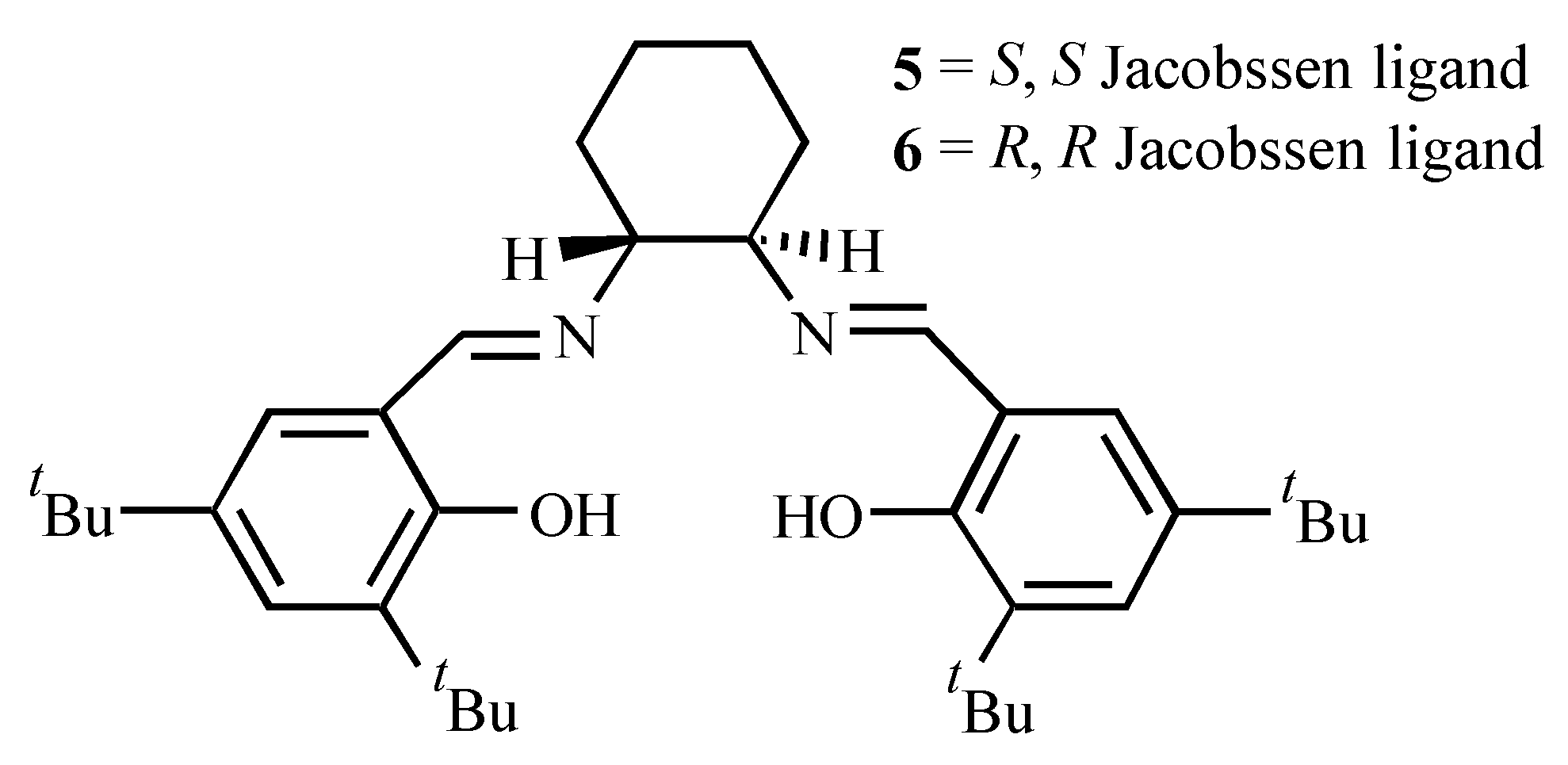
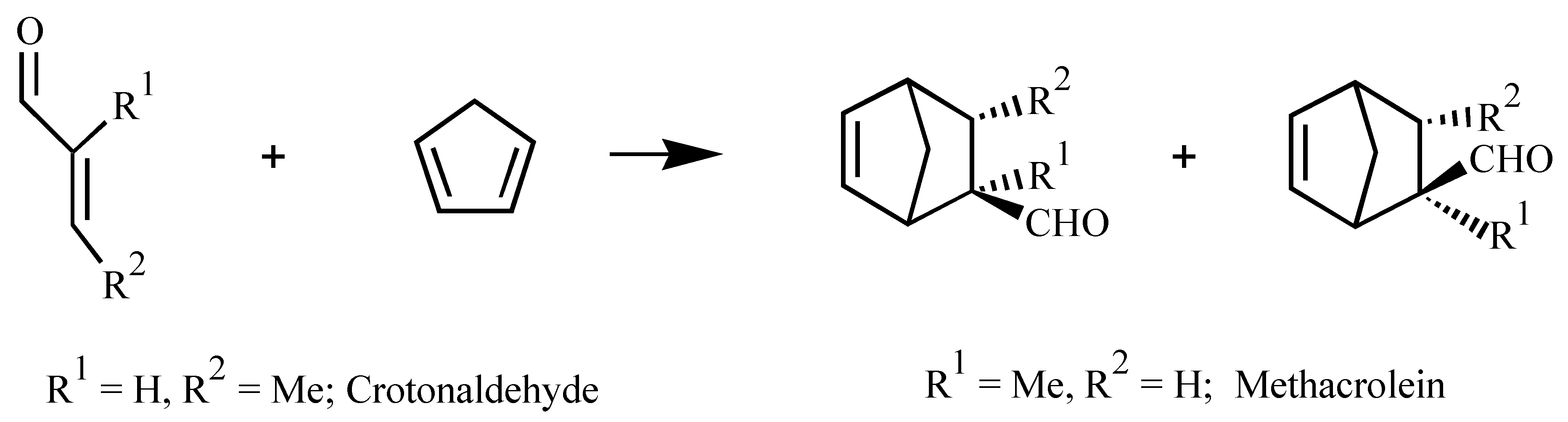
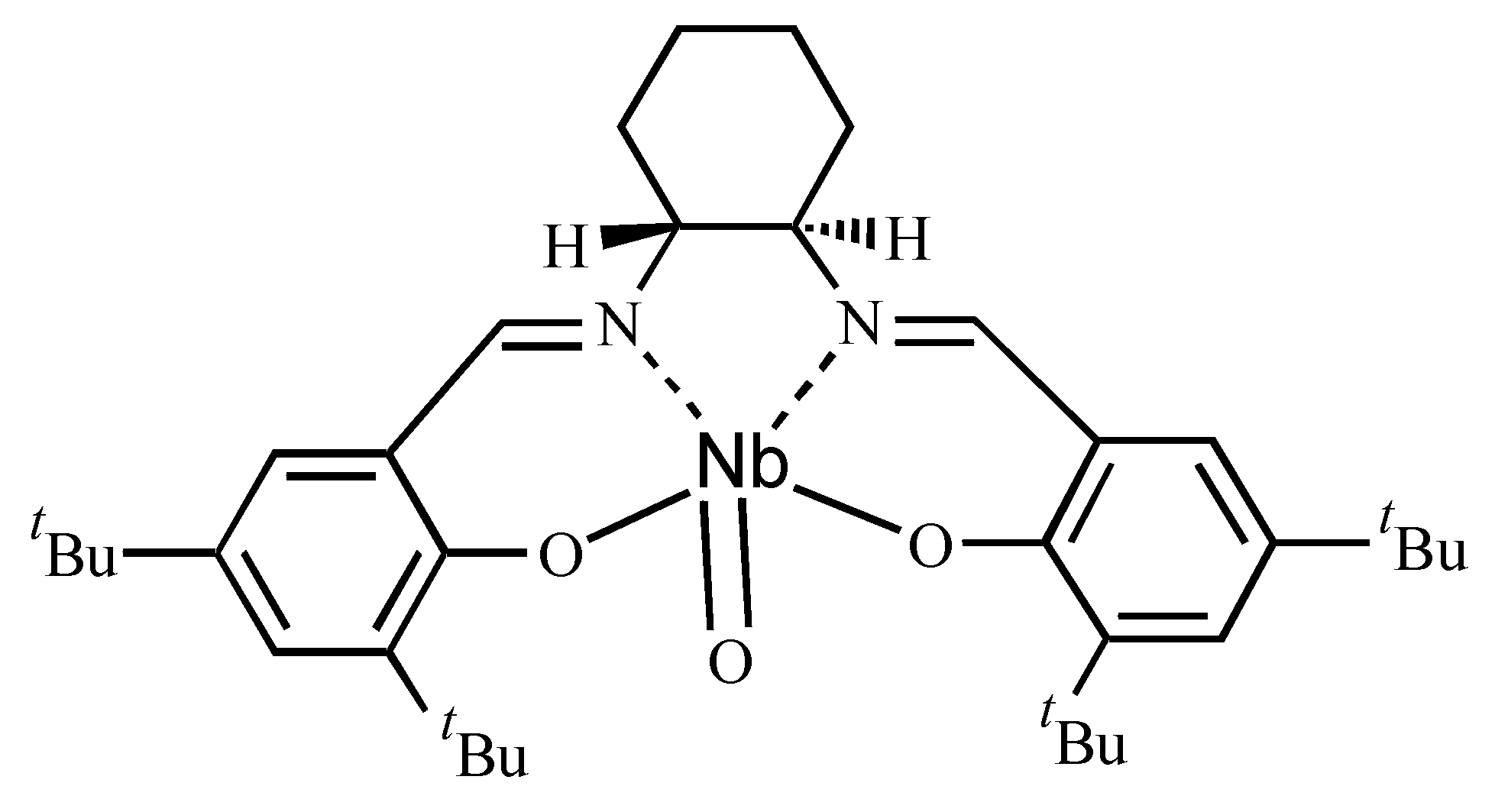
| Ligand | Dienophile | Yield (%) | Endo:Exo Ratio | Ee (%) | |||
|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | ||
| 1 | M | 12 | 36 | 7:93 | 15:85 | <3 | 10 (S) |
| C | 7 | 18 | 92:8 | 85:15 | <3 | <3 | |
| 2 | M | 40 | 87 | 10:90 | 8:92 | <3 | 10 (S) |
| C | 27 | 39 | 85:15 | 87:13 | <3 | <3 | |
| 3 | M | 68 | 70 | 11:89 | 9:91 | 11 (R) | 23 (R) |
| C | 25 | 71 | 89:11 | 88:12 | <3 | <3 | |
| 4 | M | 99a | 56 | 8:92 | 10:90 | <3 | <3 |
| C | 22 | 35 | 88:12 | 87:13 | <3 | <3 | |
| 5 | M | 67a | 86 | 9:91 | 7:93 | 40 (R) | 40 (R) |
| C | 39 | 58 | 89:11 | 76:24 | 25 (R) | 19 (R) | |
| 6 | M | 49 | 74 | 10:90 | 6:94 | 38 (S) | 42 (S) |
| C | 28 | 61 | 85:15 | 79:21 | 27 (S) | 25 (S) | |
| Ligand | Dienophile | Yield (%) | Endo:Exo Ratio | Endo:Exo Ratio | |||
|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | ||
| 1 | M | n.r. | 11 | 25:75 | <3 | ||
| C | n.r. | 7 | 83:17 | <3 | |||
| 2 | M | n.r. | 10 | 16:84 | |||
| C | n.r. | 8 | 89:11 | <3 | |||
| 3 | M | 33a | 84a | 9:91 | 12:88 | 43 (R) | 31 (R) |
| C | 15 | 18 | 82:18 | 87:13 | 27 (R) | 20 (R) | |
| 4 | M | 19 | 84 | 14:86 | 2:98 | <3 | <3 |
| C | 25 | 45 | 84:16 | 80:20 | <3 | <3 | |
| 5 | M | n.r. | 72a | 4:96 | 46 (R) | ||
| C | n.r. | 45a | 81:19 | 23 (R) | |||
| 6 | M | n.r. | 54 | 2:98 | 47 (S) | ||
| C | n.r. | 19 | 80:20 | 19 (S) | |||
| Metal | Ligand | Yield (%) | Endo:Exo Ratio | Ee (%) | |||
|---|---|---|---|---|---|---|---|
| A | U | A | U | A | U | ||
| Niobium | 1 | 43 | 68 | 2:98 | 7:93 | 52 (S) | 31 (S) |
| 2 | 35 | 54 | 6:94 | 6:94 | 20 (S) | <3 | |
| 3 | 41 | 44 | 2:98 | 1:99 | 35 (R) | 23 (R) | |
| 4 | 33 | 41 | 3:97 | 9:91 | 17 (S) | <3 | |
| 5 | 60 | 66 | 7:93 | 7:93 | 55 (R) | 35 (R) | |
| 6 | 46 | 34 | 8:92 | 8:92 | 48 (S) | 28 (S) | |
| Tantalum | 5 | 34 | 32 | 7:93 | 7:93 | 18 (R) | 8 (R) |
| 6 | 43 | 39 | 8:92 | 7:93 | 15 (S) | 8 (S) | |
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Howarth, J.; Gillespie, K. Lewis Acid Catalysis of the Diels–Alder Reaction Using Niobium and Tantalum Chlorides in the Presence of Coordinating Ligands. Molecules 2000, 5, 993-997. https://doi.org/10.3390/50800993
Howarth J, Gillespie K. Lewis Acid Catalysis of the Diels–Alder Reaction Using Niobium and Tantalum Chlorides in the Presence of Coordinating Ligands. Molecules. 2000; 5(8):993-997. https://doi.org/10.3390/50800993
Chicago/Turabian StyleHowarth, Joshua, and Kevin Gillespie. 2000. "Lewis Acid Catalysis of the Diels–Alder Reaction Using Niobium and Tantalum Chlorides in the Presence of Coordinating Ligands" Molecules 5, no. 8: 993-997. https://doi.org/10.3390/50800993




