A Carboxylic Acid from Ilex integra
Abstract
:Introduction
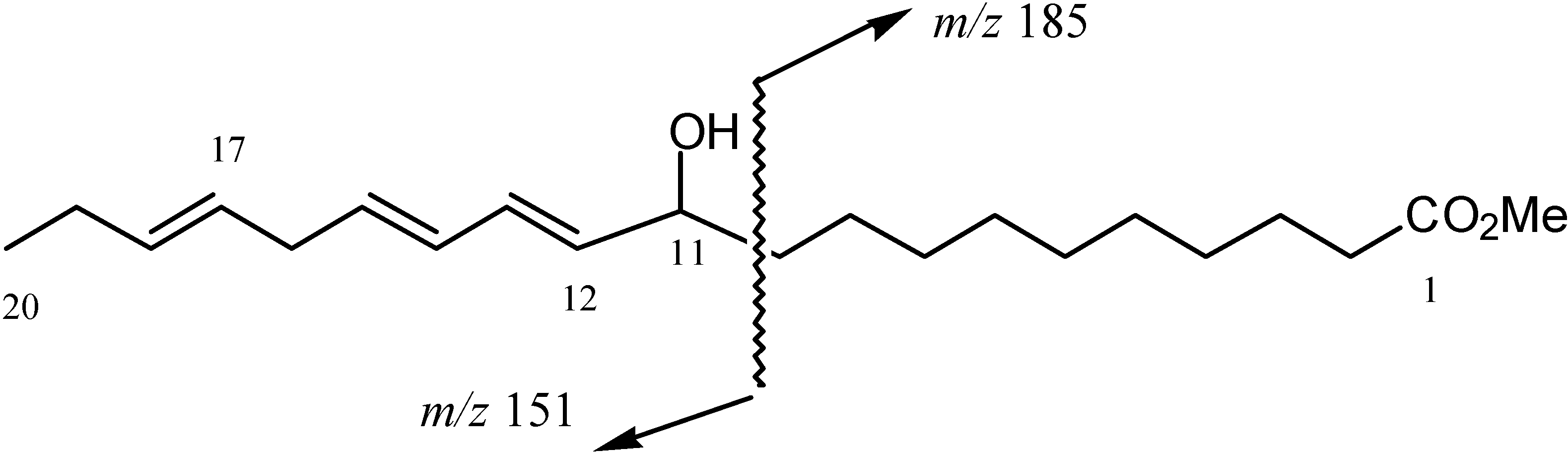
Results and Discussion

Acknowledgements
Experimental
General
Plant material
Extraction and isolation
References
- Yano, I.; Nishiizumi, C.; Yoshikawa, K.; Arihara, S. Triterpenoids saponins from Ilex integra. Phytochemistry 1993, 32, 417–420. [Google Scholar] [CrossRef]
- Haraguchi, H.; Kataoka, S.; Okamoto, S.; Hanafi, M.; Shibata, K. Antimicrobial triterpenes from Ilex integra and the mechanism of antifungal action. Phytotherapy Res. 1999, 13, 151–156. [Google Scholar] [CrossRef]
- Hirose, M.; Kosuzume, K.; Goshima, M.; Tanabe, F.; Satoh, Y.; Hagitani, A. The constituents of the seeds of Ilex integra Thunb. Part I. The components of the fatty acids of seeds of Ilex integra Thunb (Mochinoki). Yukagaku 1971, 20, 7–9. [Google Scholar]
- Sample Availability: Samples not available.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Tori, M.; Mukai, Y.; Nakashima, K.; Sono, M. A Carboxylic Acid from Ilex integra. Molecules 2003, 8, 882-885. https://doi.org/10.3390/81200882
Tori M, Mukai Y, Nakashima K, Sono M. A Carboxylic Acid from Ilex integra. Molecules. 2003; 8(12):882-885. https://doi.org/10.3390/81200882
Chicago/Turabian StyleTori, Motoo, Yoshie Mukai, Katsuyuki Nakashima, and Masakazu Sono. 2003. "A Carboxylic Acid from Ilex integra" Molecules 8, no. 12: 882-885. https://doi.org/10.3390/81200882



