Synthesis, Crystal Structure, Stacking Effect and Antibacterial Studies of a Novel Quaternary Copper (II) Complex with Quinolone
Abstract
:Introduction
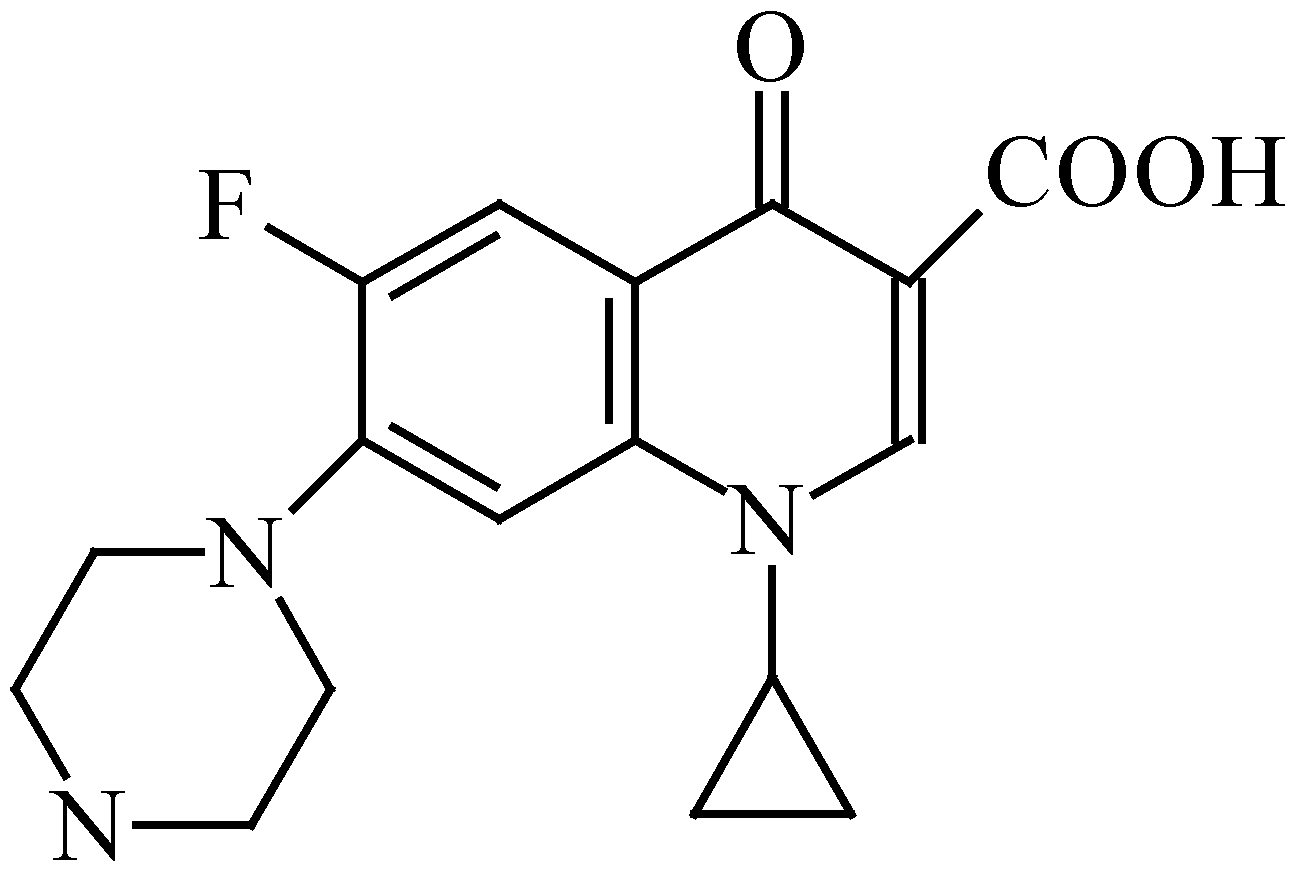
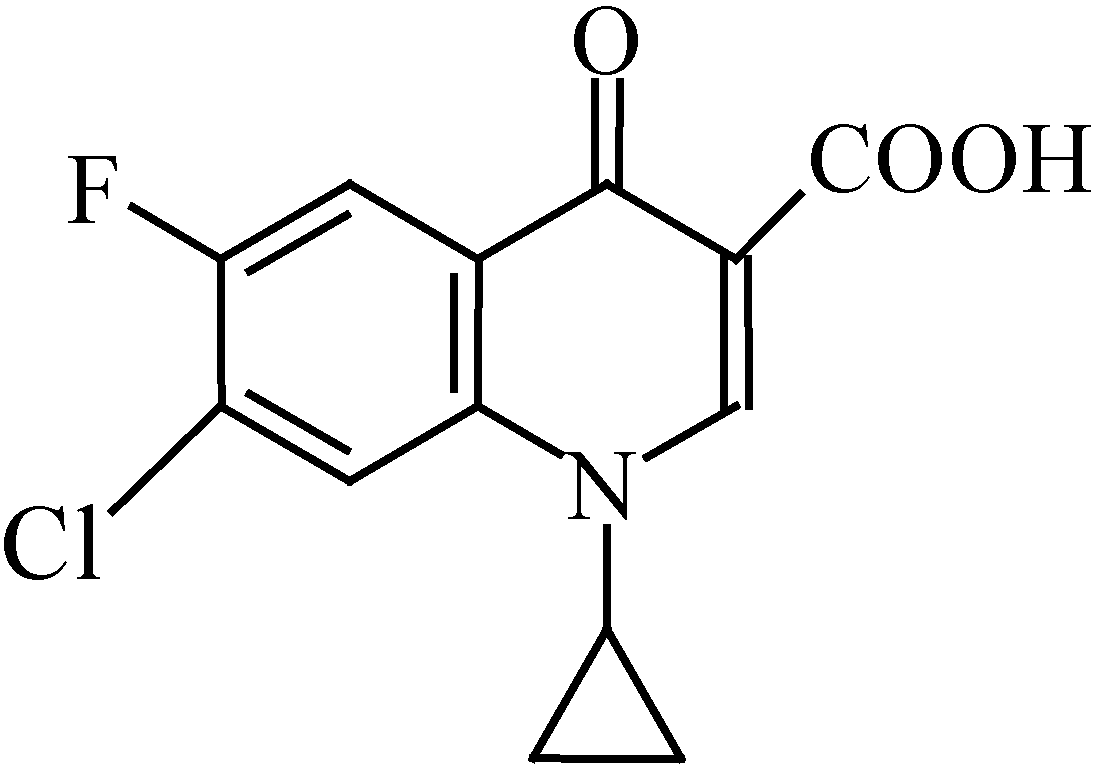
Results and Discussion
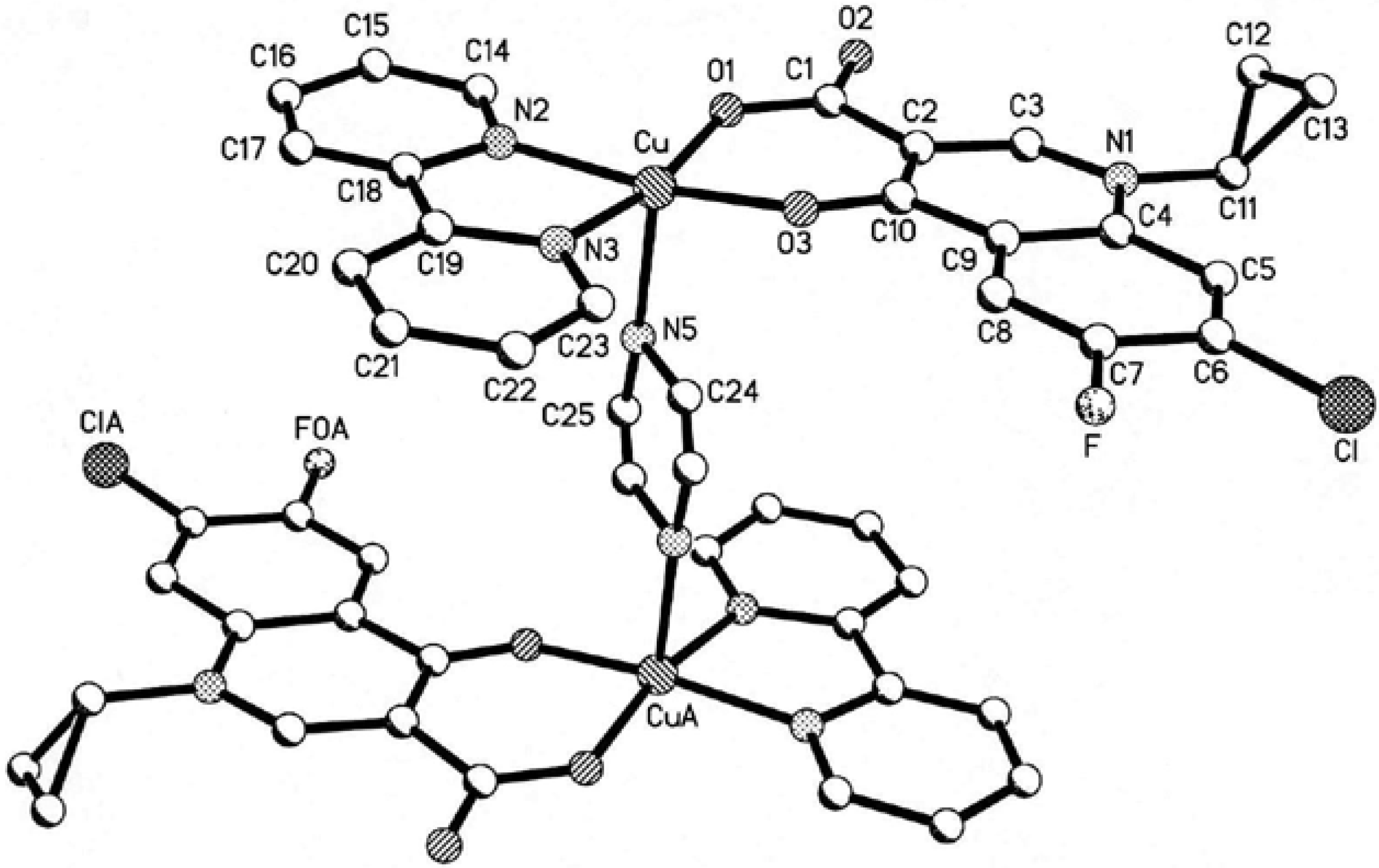
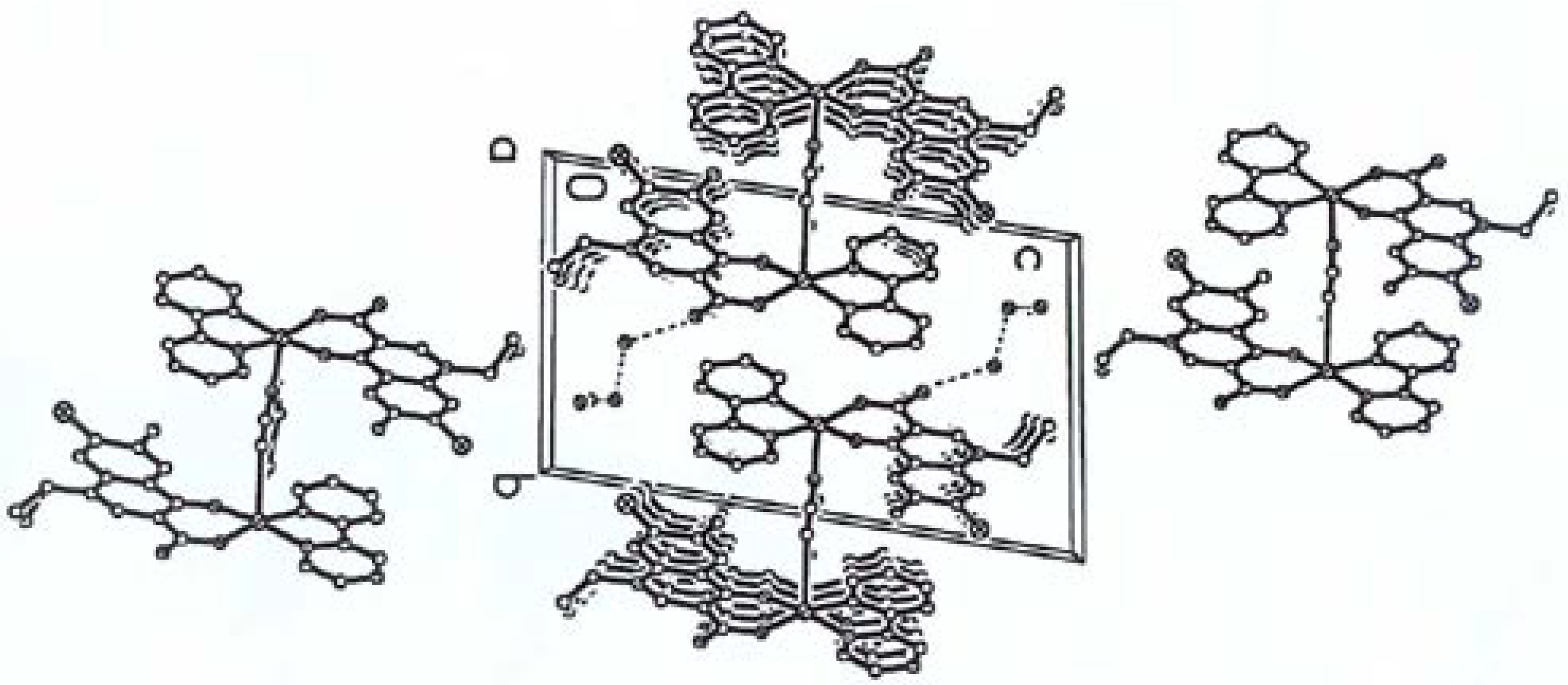
The Structure of [Cu2(cip)2(bpy)2(pip)]·6H2O [10]
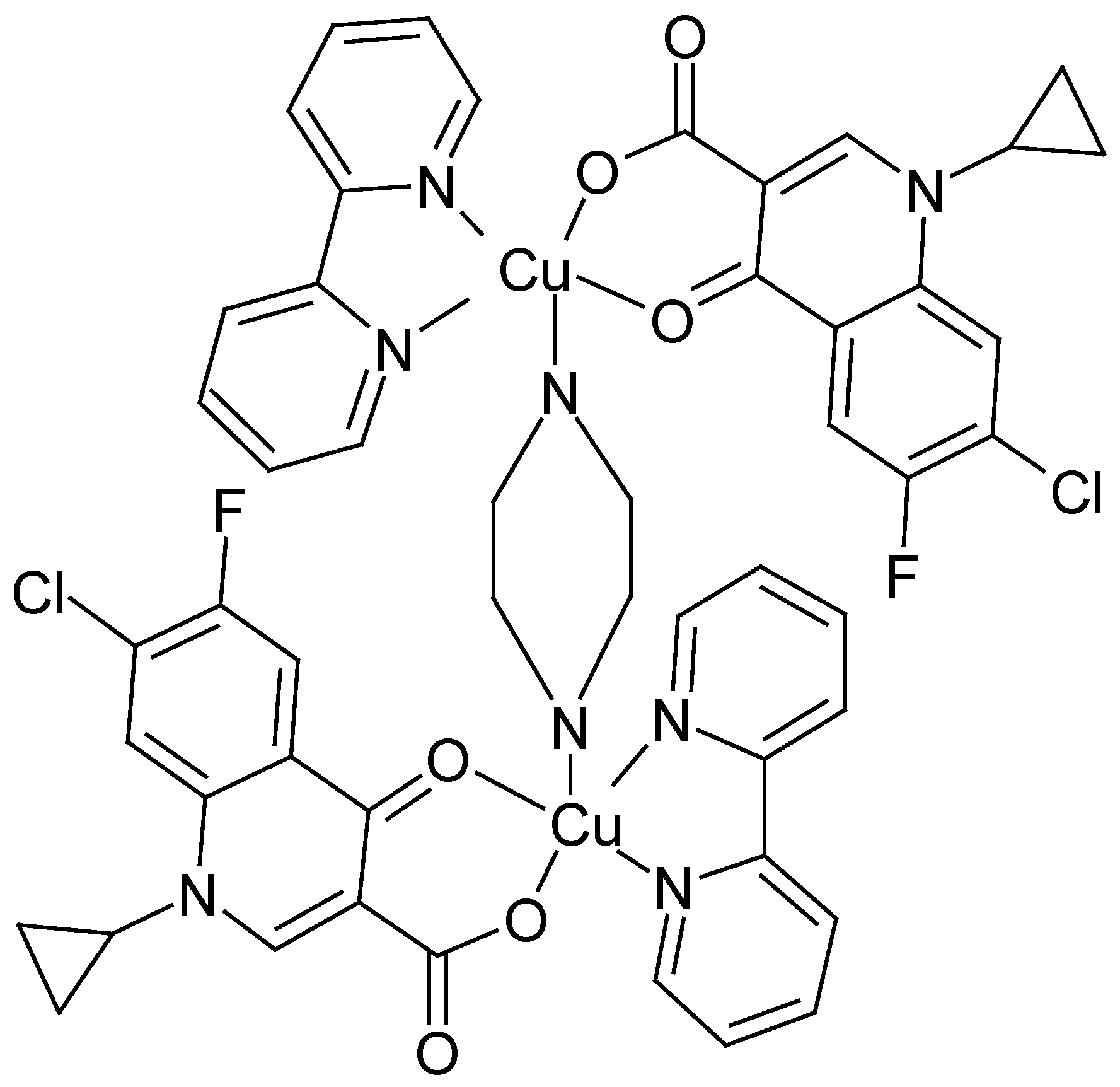
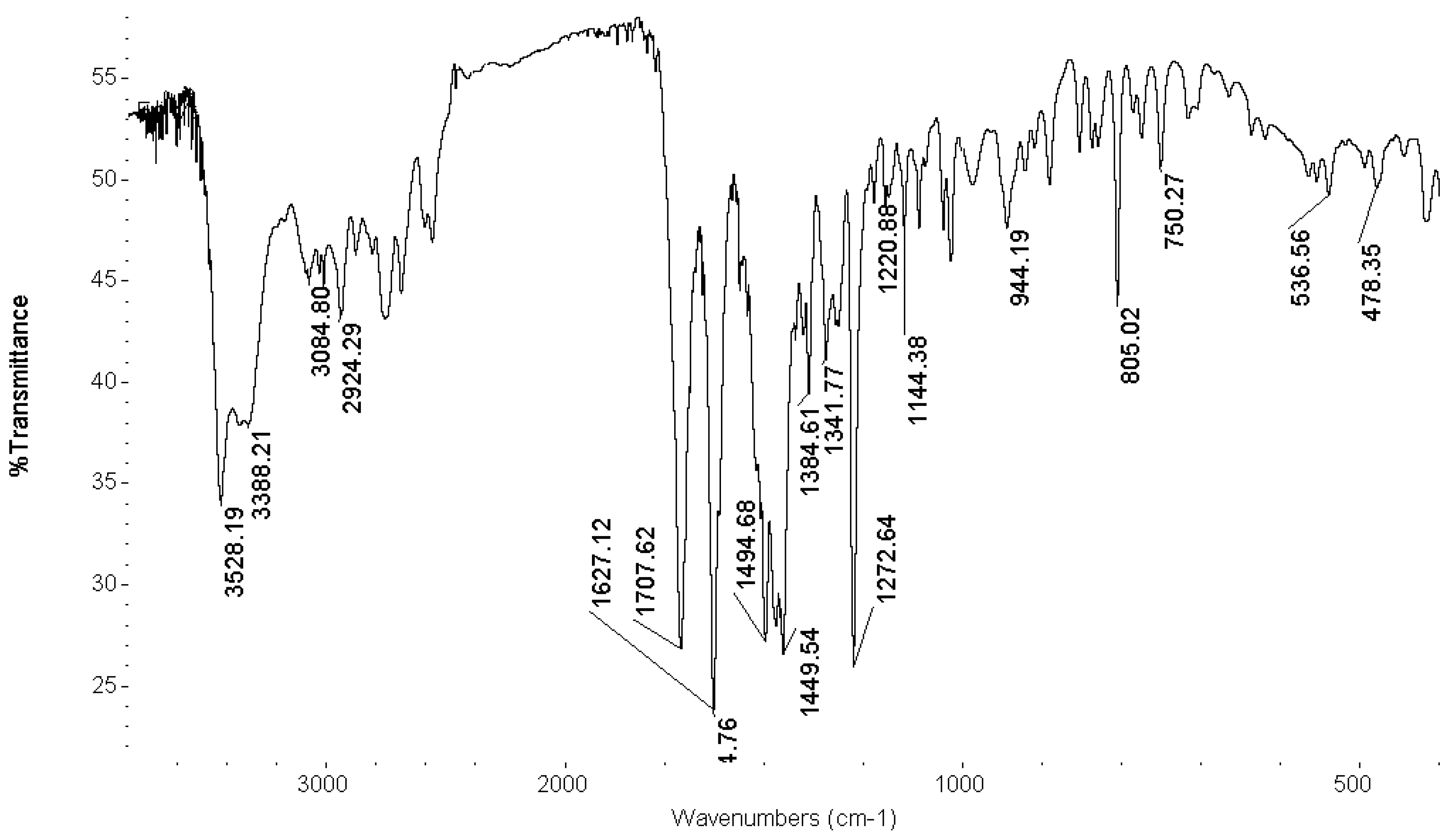
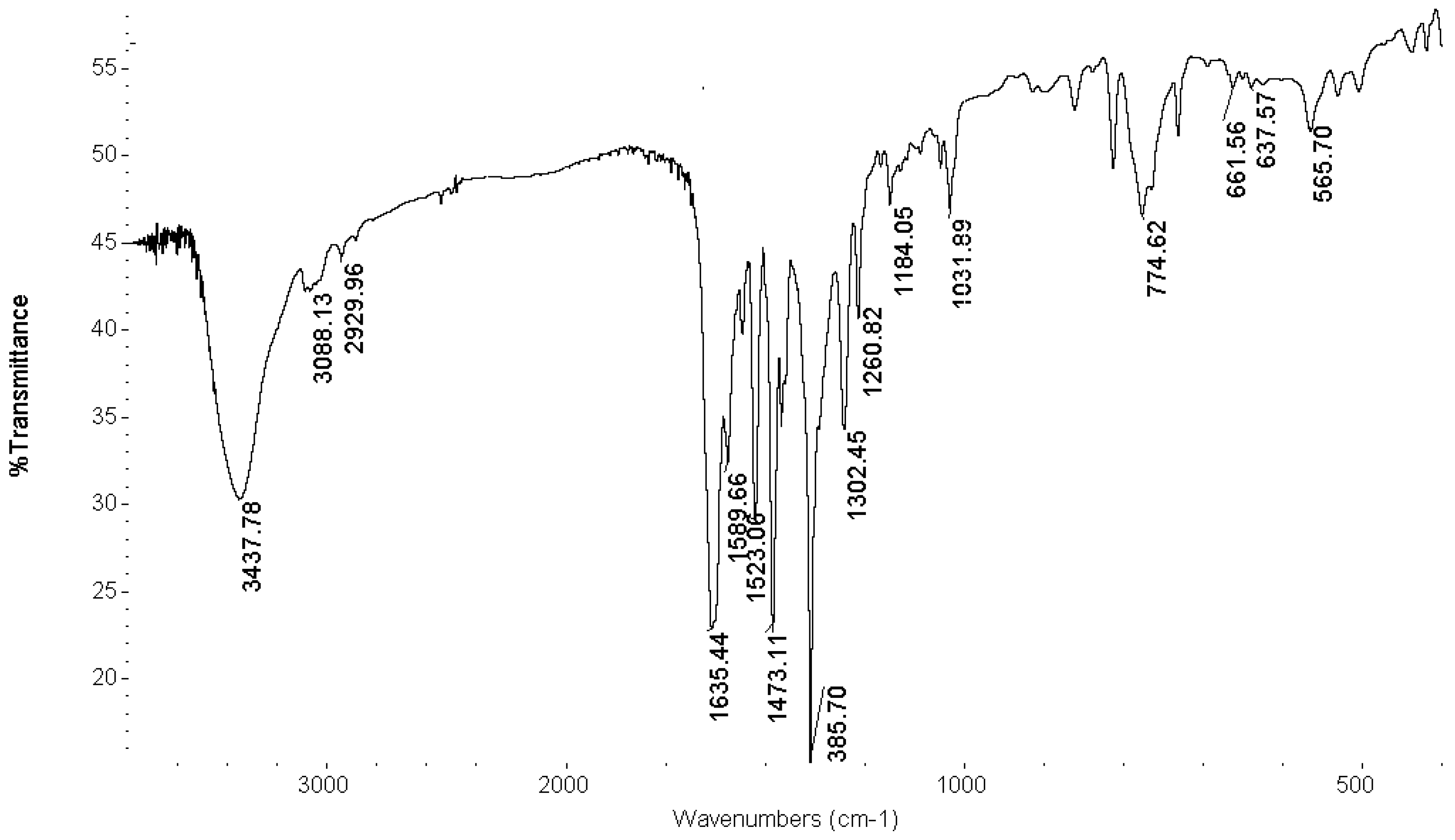
FT-IR Spectroscopy
Antibacterial Studies
| Compound | Microorganism | |||
|---|---|---|---|---|
| Staphylococcus Aureus | Micrococcus Lutens | Escherichia Coli | Pseudomonas Aeruginosa | |
| complex | <20 | >20 | <20 | >20 |
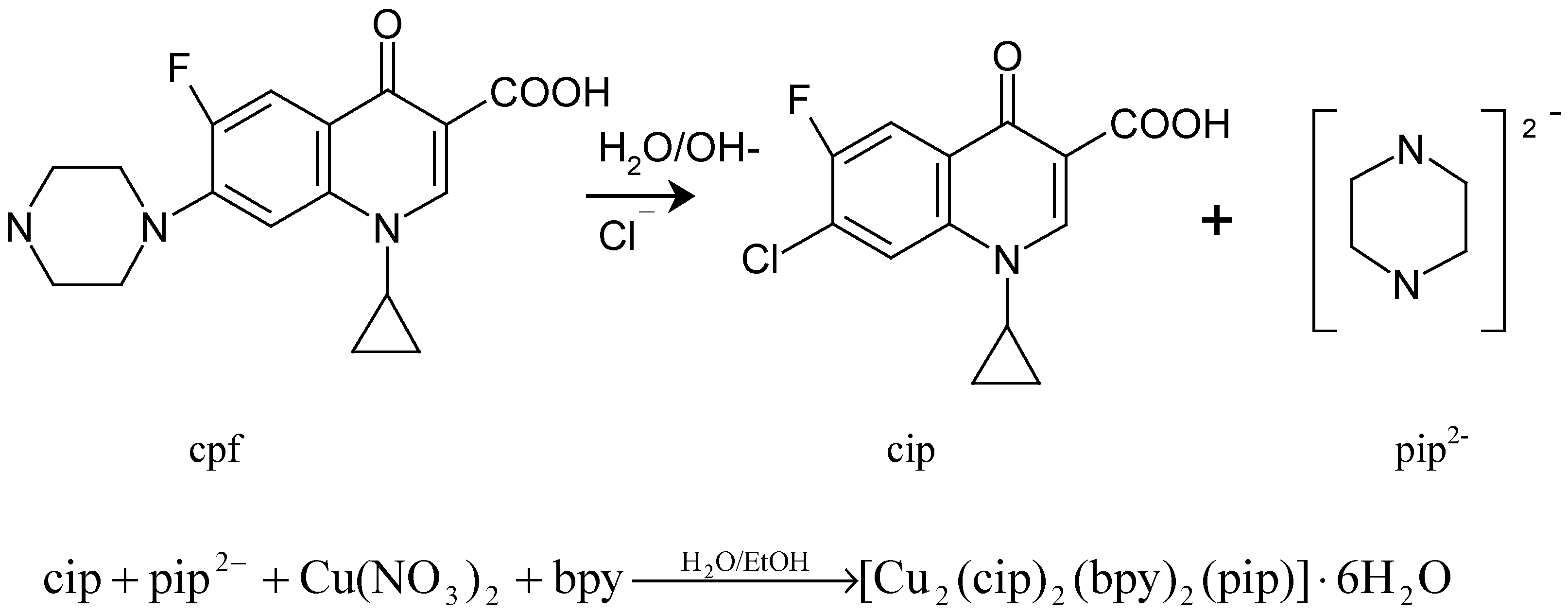
Possible synthetic reaction
Experimental
Chemicals, analyses and physical measurements
Synthesis of [Cu2(cip)2(bpy)2(pip)]·6H2O
Crystallographic Data and Structure Determination
| Empirical formula | C25 H26 Cl Cu F N4 O6 | Volume of unit cell | 1284.5(6) Å3 |
| Formula weight | 596.49 | Z | 2 |
| Temperature | 293(1)K | Density (calculated) | 1.542 g·cm-3 |
| Mo-Kα | 0.71073Å | F(000) | 614 |
| μ(Mo-Kα) | 12.13cm-1 | Crystal size | 0.20×0.20×0.30mm |
| Crystal system | Triclinic | θ range | 1.95 ° to 25.50° |
| Space group | P-1 | Reflections collected | 6702 |
| Unit-cell dimensions | independent reflections | 4697(Rint=0.073) | |
| a = 6.874(2) Å , α=80.071(6) ° | Goodness of fit on F2 | 0.897 | |
| b =10.761(3) Å , β = 85.253(6) ° | Final R indices[I>2.00σ (I)] | R1=0.0776 | |
| c = 17.976(5) Å , γ=79.109(6) ° | wr2=0.2235 | ||
| Max. peak in final diff. Map | 0.881e-1/ Å3 | Min. peak in final diff. Map | -0.437e-1/ Å3 |
Antibacterial Susceptibility Tests
Acknowledgements
References
- Sorenson, J.R.J. Copper Chelates as Possible Active Forms of the Anti-arthritic Agents. J. Med. Chem. 1976, 19, 135–148. [Google Scholar]
- Brown, D.H.; Lewis, A.J.; Smith, W. E.; Teape, J.W. Anti-Inflammatory Effects of Some Copper Complexes. J. Med. Chem. 1980, 23, 729–734. [Google Scholar]
- Williams, D.R. The Metals of Life; Van Nostrand Reinhold: London, 1971. [Google Scholar]
- Ruiz, M.; Perello, L.; Ortiz, R.; Castineiras, A.; Maichlemossmer, C.; Canton, E. Synthesis, Characterization, and Crystal-Structure of Cu(Cinoxacinate)2·2H2O Complex. A Square-Planar CuO4 Chromophore, Antibacterial Studies. J. Inorg. Biochem. 1995, 59, 801–810. [Google Scholar]
- Castillo-Blum, S.E.; Barba-Behrens, N. Coordination Chemistry of Some Biologically Active Ligands. Coord. Chem. Rev. 2000, 196, 3–30. [Google Scholar]
- Turel, I.; Leban, I.; Bukovec, N. Crystal Structure and Characterization of the Bismuth (III) Compound with Quinolone Family Member (Ciprofloxacin). Antibacterial Study. J. Inorg. Biochem. 1997, 66, 241–245. [Google Scholar]
- Turel, I.; Golic, L.; Bukovec, P.; Gubina, M. Antibacterial Tests of Bismuth (III) -Quinolone (Ciprofloxacin, cf) Compounds against Helicobacter Pylori and Some Other Bacteria. Crystal Structure of (cfH2)2[Bi2Cl10] · 4H2O. J. Inorg. Biochem. 1998, 71, 53–60. [Google Scholar]
- Yang, P.; Li, J.B.; Tian, Y.N.; Yu, K.B. Synthesis and Crystal Structure of Rare Earth Complex with Ciprofloxacin. Chin. Chem. Let. 1999, 10, 879–880. [Google Scholar]
- Wang, G.P.; Cai, G.Q.; Zhu, L.G. Synthesis and Crystal Structure of Fluroquinolone Complex of Copper with Two-Dimension Network. Chin. J. Inorg. Chem. 2000, 6, 987–990. [Google Scholar]
- CCDC 191087 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via the URL http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223 336033; e-mail: [email protected]).
- Cromer, D.T.; Waber, J.T. International Tables for X-Ray Crystallography; The Kynoch Press: Birmingham (U.K.), 1974; Vol. IV, Table 2.2 A. [Google Scholar]
- Wallis, S.C.; Gahan, L.R.; Charles, B.G.; Hambley, T.W.; Duckworth, P.A. Copper (II) Complexes of the Fluoroquinolone Antimicrobial Ciprofloxacin. Synthesis, X-Ray Structural Characterization, and Potentiometric Study. J. Inorg. Biochem. 1996, 62, 1–16. [Google Scholar]
- Wallis, S.C.; Gahan, L.R.; Charles, B.G.; Hambley, T.W. Synthesis and X-Ray Structural Characterization of an Iron (III) Complex of the Fluoroquinolone Antimicrobial Ciprofloxacin, [Fe(CIP)(NTA)]3·5H2O(NTA=Nitrilotriacetato). Polyhedron 1995, 14, 2835–2840. [Google Scholar]
- Berman, H.M.; Shieh, H.S. Topics in Nucleic Acid Structure; Neidle, S., Ed.; MacMillan Publishers LTD.: London (U.K.), 1981; pp. 17–32. [Google Scholar]
- Voet, D.; Voet, J.G. Biochemistry; John Wiley & Sons: New York, 1990; p. 795. [Google Scholar]
- Mendoza Diaz, G.; Martinez Aguilera, L.M.R.; Moreno Esparza, R.; Pannell, K.H.; Cervantes Lee, F. Some Mixed-Ligand Complexes of Copper (II) with Drugs of the Quinolone Family and (N-N) Donors - Crystal-Structure of [Cu(Phen)(Cnx)(H2O)]NO3·H2O. J. Inorg. Biochem. 1993, 50, 65–78. [Google Scholar]
- Chun-che, T.; Shri-C, J.; Sobell, M. H. Proc. Natl. Acad. Sci. USA 1975, 62, 628.
- Shen, L.L.; Pernet, A.G.; Odonnell, T.J.; Rosen, T.; Chu, D.W.T.; Sharma, P.N.; Mitscher, L.A.; Cooper, C.S. Mechanism of Inhibition of DNA Gyrase by Quinolone Antibacterials - A Cooperative Drug-DNA Binding Model. Biochemistry. 1989, 28, 3886–3894. [Google Scholar]
- Wang, G.P.; Zhu, L.G. Synthesis and Crystal Structure of a New Copper (II) Complex Containing Fluoroquinolone. In Frontiers of Solid State Chemistry; Feng, S.H., Chen, J.S., Eds.; World Scientific: Singapore, 2002; pp. 327–331. [Google Scholar]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules; 3rd Edition; Chapman and Hall: London (U.K.), 1975. [Google Scholar]
- Macias, B.; Villa, M.V.; Rubio, I.; Castineiras, A.; Borras, J. Complexes of Ni(II) and Cu(II) with Ofloxacin, Crystal Structure of a New Cu(II) Ofloxacin Complex. J. Inorg. Biochem. 2001, 84, 163–170. [Google Scholar]
- Wang, G.P.; Lei, Q.F. Synthesis, Electron Paramagnetic Resonance Properties and Antibacterial Studies of Copper (II) Complex Containing Norfloxacin. J. Zhejiang University (Science Edition) 2003, 30, 92–96. [Google Scholar]
- Sample availability: Samples are available from the corresponding author.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Wu, G.; Wang, G.; Fu, X.; Zhu, L. Synthesis, Crystal Structure, Stacking Effect and Antibacterial Studies of a Novel Quaternary Copper (II) Complex with Quinolone. Molecules 2003, 8, 287-296. https://doi.org/10.3390/80200287
Wu G, Wang G, Fu X, Zhu L. Synthesis, Crystal Structure, Stacking Effect and Antibacterial Studies of a Novel Quaternary Copper (II) Complex with Quinolone. Molecules. 2003; 8(2):287-296. https://doi.org/10.3390/80200287
Chicago/Turabian StyleWu, Guangguo, Guoping Wang, Xuchun Fu, and Longguan Zhu. 2003. "Synthesis, Crystal Structure, Stacking Effect and Antibacterial Studies of a Novel Quaternary Copper (II) Complex with Quinolone" Molecules 8, no. 2: 287-296. https://doi.org/10.3390/80200287




