Dipolar Cycloaddition Reactions with Quinazolinones: A New Route for the Synthesis of Several Annelated Pyrrolo- and Pyridazinoquinazoline Derivatives
Abstract
:Introduction
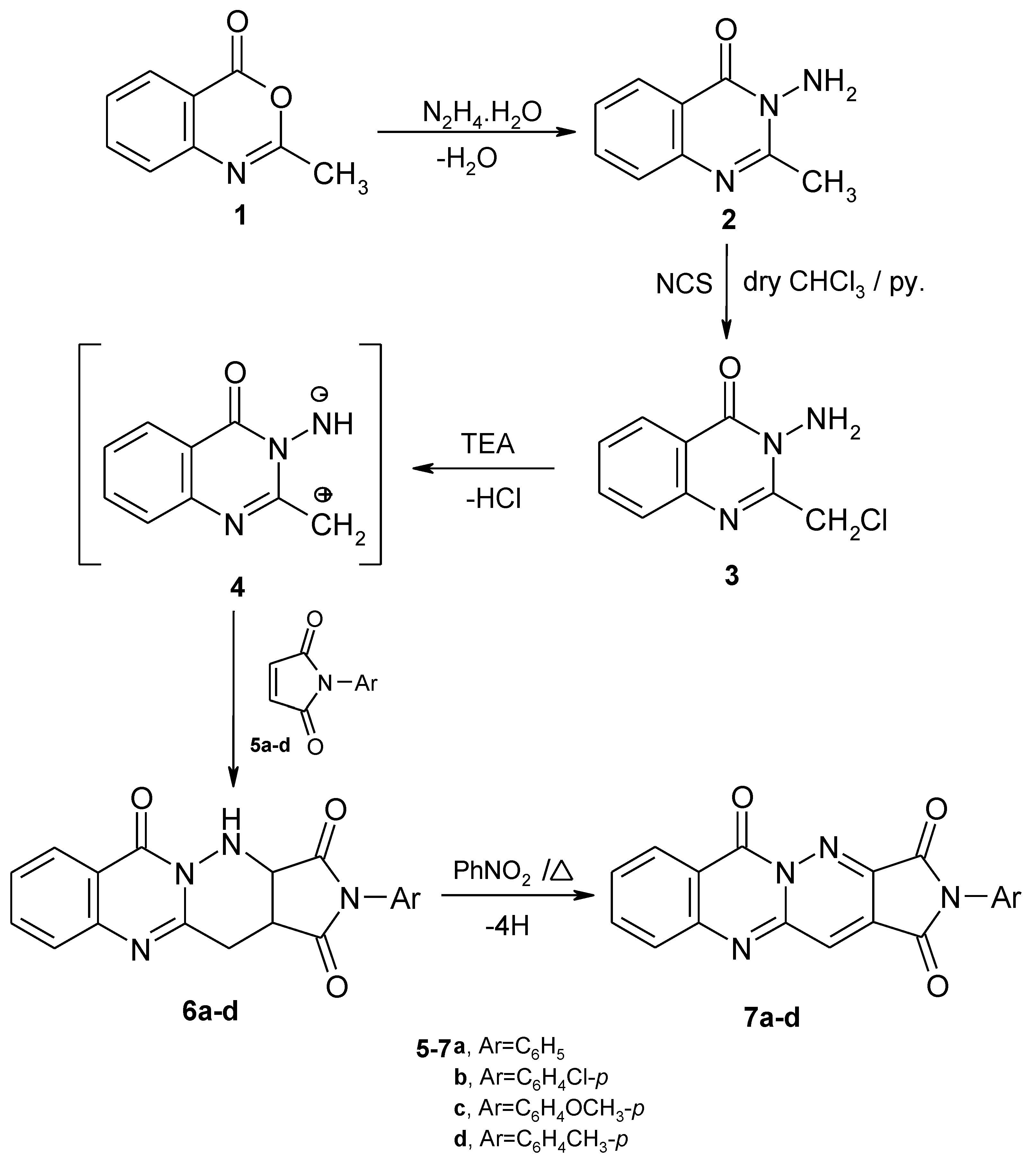
Results and Discussion

Experimental
General
Synthesis of 3-amino-2-chloromethyl-4(3H)-quinazolinone (3) [26].
General Procedure for the Synthesis of 2-aryl-3a,4,12,12a-tetrahydropyrrolo[3',4':4,3]-pyridazino-[6,1-b]quinazoline-1,3,6-triones (6a–d), 2-aryl-10-oxopyridazino[6,1-b]quinazoline-3-thio-carboxamides (10a–d) and 2-aryl-3-nitro-1,2,3,4-tetrahydropyridazino[6,1-b]quinazolin-10-ones (12a–d).
General Procedure for the Synthesis of 2-arylpyrrolo[3',4':4,3]pyridazino[6,1-b]quinazoline-1,3,6-triones 7a–d and 2-aryl-3-nitropyridazino[6,1-b]quinazolin-10-ones 13a–d
Acknowledgements
References
- Bartroli, J.; Turmo, E.; Alguero, M.; Boncompte, E.; Vericat, M. L.; Conte, L.; Ramis, J.; Merlos, M.; Garcia-Rafanell, J.; Forn, J. J. Med. Chem. 1998, 41, 1869. [CrossRef]
- Shiba, S. A.; El-Khamry, A. A.; Shaban, M. E.; Atia, K. S. Pharmazie 1997, 52, 189.
- Abdel-Hamid, S. G. J. Ind. Chem. Soc. 1997, 74, 613.
- Barker, A. J. Eur. Pat. 1995, 635498, [Chem. Abstr., 1995, 122, 214099].
- Bekhit, A. A.; Khalil, M. A. Pharmazie 1998, 53, 539.
- Gursoy, A.; Karali, N. Farmaco. 1995, 50, 857. [Google Scholar]
- Nawrocka, W.; Zimecki, M. Arch. Pharm. 1997, 330, 399.
- Kurogi, Y.; Inoue, Y.; Tsutsumi, K.; Nakamura, S.; Nagao, K.; Yoshitsugu, H.; Tsuda, Y. J. Med. Chem. 1996, 39, 143. [CrossRef]
- Hamel, E.; Lin, C. M.; Plowman, J.; Wang, H. K.; Lee, K. H.; Paull, K. D. Bioorg. Pharm. 1996, 51, 53.
- Terashima, K.; Shimamura, H.; Kawase, A.; Tanaka, Y.; Tanimura, T.; Kamisaki, T.; Ishizuka, Y.; Sato, M. Chem. Pharm. Bull. 1995, 43, 2021. [CrossRef]
- Raffa, D.; Dailone, G.; Maggio, B.; Sehillaci, D.; Plescia, F. Arch. Pharm. 1999, 332, 317.
- Baek, D. J.; Park, Y. K.; Heo, H. I.; Lee, M. H.; Yang, Z. Y.; Chio, M. H. Bioorg. Med. Chem. Lett. 1998, 8, 3287. [CrossRef]
- Griffin, R. J.; Srinivasan, S.; Bowman, K.; Calvert, A. H.; Curtin, N. J.; Newell, D. R.; Pemberton, L. C.; Golding, B. T. J. Med. Chem. 1998, 41, 5256. [CrossRef]
- Narimatsu, A.; Katida, J.; Satoh, N.; Suzuki, R.; Kobayashi, M.
- Coudert, P.; Rubat, C.; Couquelet, J. Eur. J. Med. Chem. 1989, 24, 551. [CrossRef]
- Strappaghetti, G.; Corsano, S.; Barbaro, R.; Lucacchini, A.; Giannaccini, G.; Betti, L. J. Med. Chem. 1998, 33, 501.
- Corsano, S.; Strappaghetti, G.; Scapicchi, R.; Lucacchini, A.; Senatore, G. Arch. Pharm. 1995, 328, 654.
- Mohareb, R. M.; Ghabrial, S. S.; Wardakhan, W. W. Phosphorus, Sulfur and Silicon 1998, 134/135, 119.
- Sherif, S. M.; Mohareb, R. M.; Shams, H. Z.; Gaber, H. M. M. J. Chem. Res. (S) 1995, 434, (M), 1995, 2658.
- Abdel-Hamid, A. O.; Ghabrial, S. S.; Zaki, M. Y.; Ramadan, N. Arch. Pharm. 1992, 329, 205.
- Abdel-Hamid, A. O.; Attaby, F. A.; Khalifa, F. A.; Ghabrial, S. S. Arch. Pharm. Res. 1992, 15, 14.
- Ghabrial, S. S.; Thomsen, I.; Torssell, K. B. G. Acta Chem. Scand. 1987, 41B, 426. [CrossRef]
- Ghabrial, S. S.; Zaki, M. Y. J. Chem. Res. (S) 1997, 234, (M), 1997, 1560.
- Ghabrial, S. S.; Abdel-Hamid, A. O. Arch. Pharm. 1987, 320, 1281.
- Bogert, M. T.; Cortner, R. A. J. Am. Chem. Soc. 1909, 31, 943. [CrossRef]
- Fetter, J.; Czuppon, T.; Hornyak, G.; Feller, A. Tetrahedron 1991, 47, 9393.
- Bellamy, L. J. The Infrared Spectra of Complex Molecules; John Wiley & Sons, Inc.: New York, 1958; pp. 221 and 229. [Google Scholar]
- Bogert, M. T.; Seil, H. A. J. Am. Chem. Soc. 1907, 29, 529.
- Searle, N. E. U. S. Pat. 1948, 2 444 536, [Chem. Abstr., 1948, 43, 7340].
- Vogel, A. I. A Textbook of Practical Organic Chemistry, 4th ed.; Longman: London, 1980; pp. 673 and 796. [Google Scholar]
- Sample Availability: Not available
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ghabrial, S.S.; Gaber, H.M. Dipolar Cycloaddition Reactions with Quinazolinones: A New Route for the Synthesis of Several Annelated Pyrrolo- and Pyridazinoquinazoline Derivatives. Molecules 2003, 8, 401-410. https://doi.org/10.3390/80500401
Ghabrial SS, Gaber HM. Dipolar Cycloaddition Reactions with Quinazolinones: A New Route for the Synthesis of Several Annelated Pyrrolo- and Pyridazinoquinazoline Derivatives. Molecules. 2003; 8(5):401-410. https://doi.org/10.3390/80500401
Chicago/Turabian StyleGhabrial, Sami S., and Hatem M. Gaber. 2003. "Dipolar Cycloaddition Reactions with Quinazolinones: A New Route for the Synthesis of Several Annelated Pyrrolo- and Pyridazinoquinazoline Derivatives" Molecules 8, no. 5: 401-410. https://doi.org/10.3390/80500401



