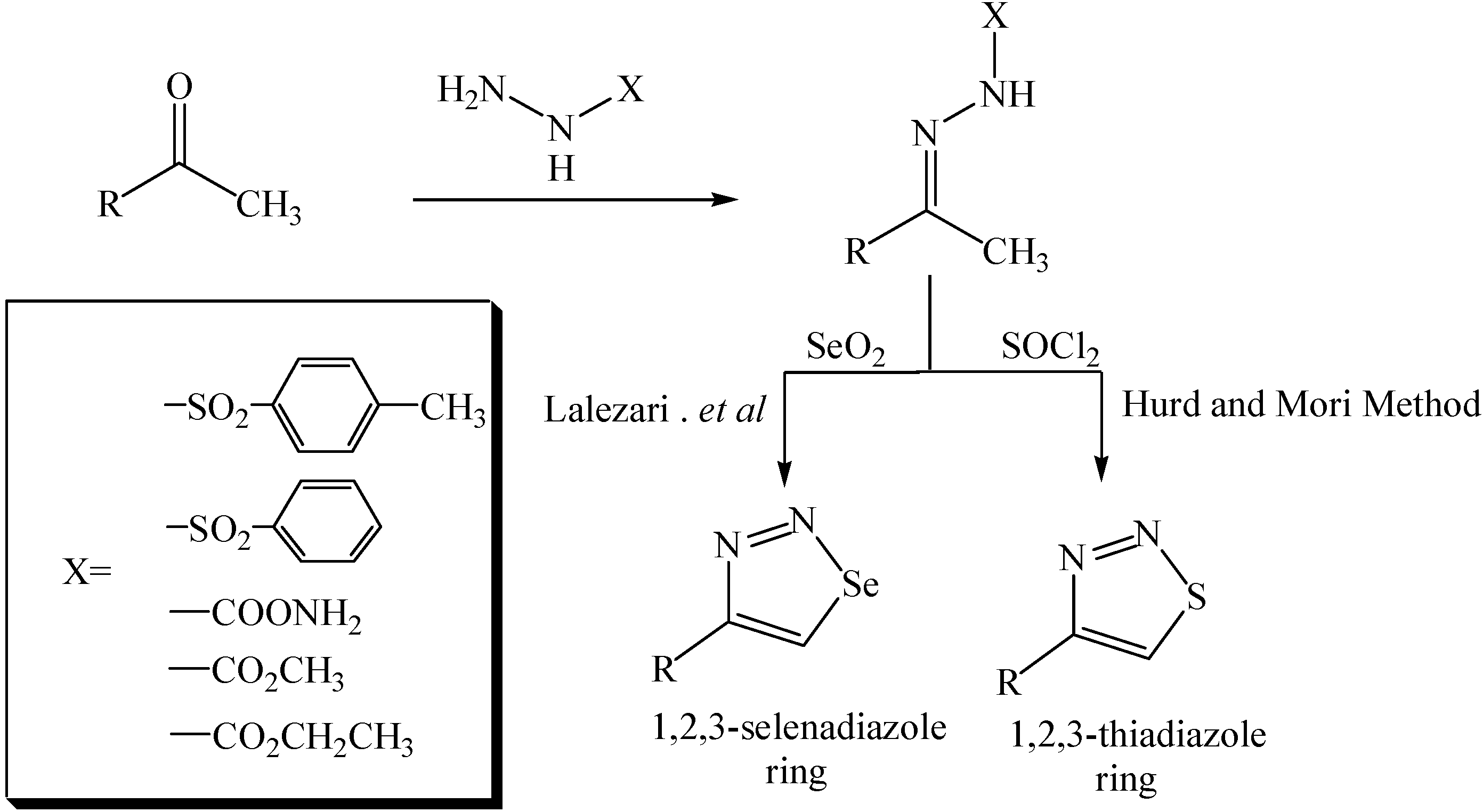
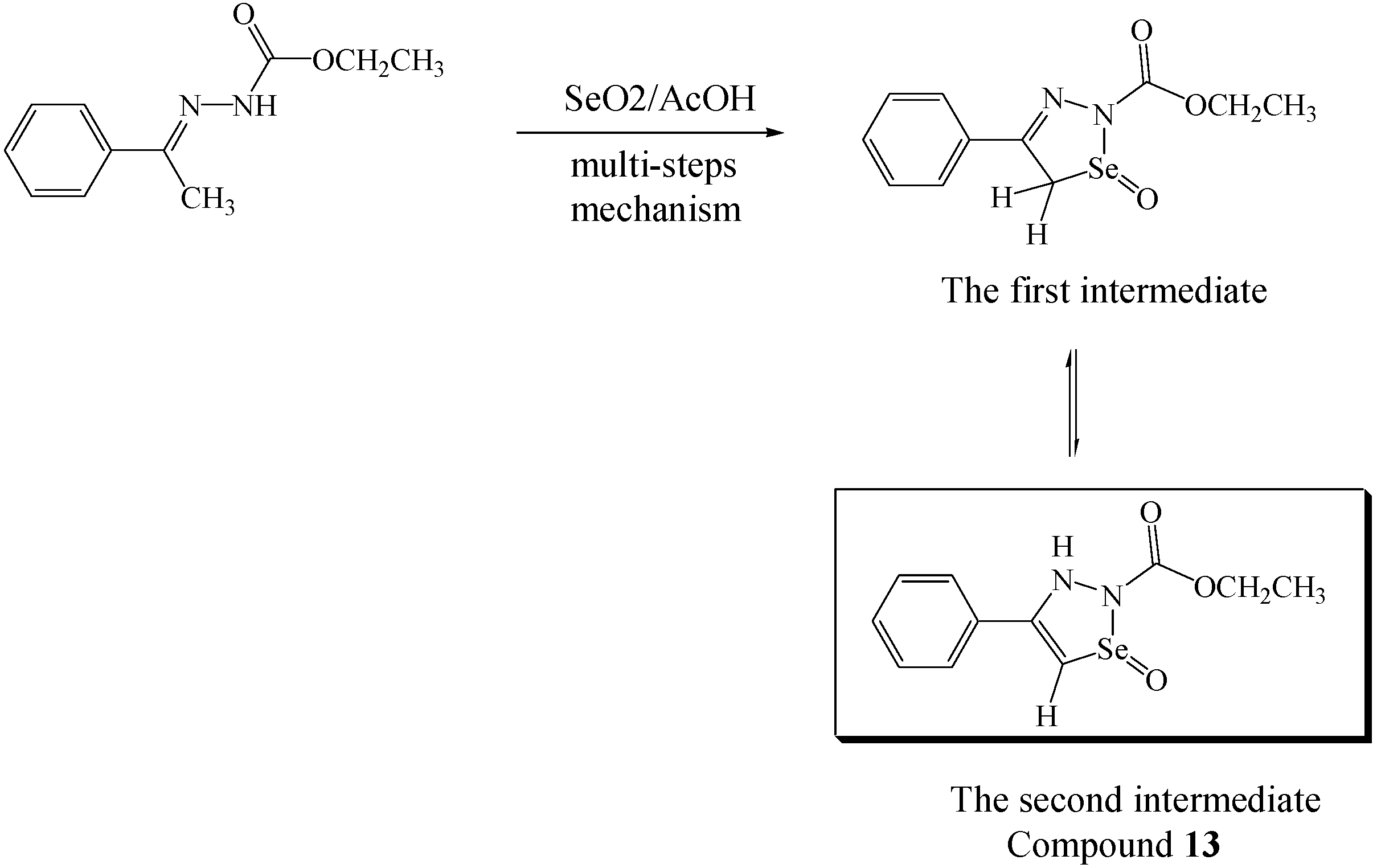
Experimental
General
The solvents used were purified by standard procedures. The melting points (m.p) were determined on an Electrothermal digital melting point apparatus and uncorrected. Infrared (IR) spectra of pure substances were recorded for KBr pellets using a NICOLET 410 FT-IR spectrometer (ν in cm-1). The 1H- and 13C-NMR spectra were recorded on Bruker AM 400 and AC200 spectrometers in CDCl3 or DMSO-d6. The spectral data are reported in delta (δ) units relative to the TMS reference peak. The mass spectra were registered using MAT CH7A (Varian, EI: 70eV Ionizing energy, electron ionization) and MAT95 (Finnigan, FD: 5kV Ionizing energy, field desorption) instruments. The signals are given as m/z with the relative intensity between brackets. Elemental analyses were performed in the analytical laboratory of the Institute of Organic Chemistry of University of Mainz, Germany. D‑(+)-camphor, acetyl ferrocene, diacetyl ferrocene, p-hydroxyacetophenone, acetophenone, ethyl hydrazine carboxylate, semicarbazide hydrochloride and sodium acetate were obtained from Aldrich.
General procedure for the preparation of semicarbazones 7, 10, 11 and 12.
A mixture of semicarbazide hydrochloride (1.00 equivalent) and sodium acetate (1.00 equivalent) was dissolved in absolute ethanol (45 mL). The mixture was heated for 15 min under reflux, then filtered while hot to remove precipitated sodium chloride. The filtrate was then mixed with p‑hydroxyacetophenone (2, 0.95 equivalents), D-(+)-camphor (3, 0.95 equivalents), acetyl ferrocene (4, 0.95 equivalents) or diacetyl ferrocene (5, 0.45 equivalents), respectively, and the resulting mixtures were heated to reflux, a few drops of concentrated hydrochloric acid were added and heating under reflux with continuous removal of the generated water was continued overnight. The solvent was removed under vacuum and the residue was washed with diethyl ether or chloroform.
N'-[1-(4-Hydroxyphenyl)-ethylidene] semicarbazone (7). White solid powder, yield = quantitative; IR: ν 3448, 3220, 1667, 1572, 1411, 1351 cm-1; 1H-NMR (DMSO-d6): δ 2.16 (s, 3H, CH3), 6.98/7.65 (AA′BB′, 4H, aromatic H), 9.97 (s, 1H, N-H); 13C-NMR (DMSO-d6): δ 14.2 (CH3), 114.8/127.6 (CH, phenylene), 131.3/158.7 (Cq, phenylene), 148.9 (CN), 154.3 (CO); MS (m/z, %): 193 (M+, 100%); Anal. Calcd. for C9H11N3O2: C, 55.95; H, 5.74; N, 21.75. Found: C, 55.91; H, 5.68; N, 21.81.
N'-(1,7,7-Trimethylbicyclo[2.2.1]hept-2-ylidene) semicarbazone (10). Pale white-green solid, yield = 74%; IR: ν 3462, 3199, 2949, 1688, 1585, 1489 cm-1; 1H-NMR (DMSO-d6): δ 0.65 (s, 3H, C10), 0.85 (s, 3H at C9), 0.91 (s, 3H at C8),1.23/1.72 (2m, 4H at C5, C6), 1.89 (d, 2H, C3), 2.28 (m, 1H at C4), 5.14 (s, 2H, NH2), 8.76 (s, 1H, N-H); 13C-NMR (DMSO-d6): δ 54.84 (C1), 157.48 (C2), 34.18 (C3), 43.47 (C4), 26.99 (C5), 32.56 (C6), 47.53 (C7), 19.32 (C8), 18.62 (C9), 11.34 (C10) and 161.74 (C11); MS (m/z, %): 209 (M+, 100%); Anal. Calcd. for C11H19N3O: C, 63.13; H, 9.15; N, 20.08. Found: C, 63.05; H, 9.10; N, 19.90.
Acetyl ferrocene semicarbazone (11). Reddish orange solid, yield = 70% yield; IR: ν 3468, 3192, 3096, 1700, 1572, 1431 cm-1; 1H-NMR (CDCl3): δ 2.11 (s, 3H, CH3-C=N), 4.13 (s, 5H, unsubstituted cyclopentadienyl ring), 4.52 and 4.30 (2d, 4H, monosubstituted cyclopentadienyl ring), 8.38 (s, 1H, N-H); 13C-NMR (CDCl3): δ 14.14 (CH3-C=N), 147.20 (C=N), 157.54 (N-C(O)-N), 69.15, 69.80, 66.65, 83.44 (10C, cyclopentadienyl rings); MS (m/z, %) : 285 (M+, 100%); Anal. Calcd. for C13H15N3OFe: C, 54.57; H, 5.64; N, 14.69. Found: C, 54.39; H, 5.54; N, 14.52.
Diacetylferrocene semicarbazone (12). Dark reddish solid, yield = 68% yield; IR: ν 3456, 3191, 1712, 1568, 1436 cm-1; 1H-NMR (CDCl3): δ 2.14 (s, 6H, CH3-C=N), 4.59 and 4.37 (2d, 8H, monosubstituted cyclopentadienyl rings), 8.32 (s, 2H, N-H); 13C-NMR (CDCl3): δ 14.28 (2C, CH3-C=N), 147.39 (2C, C=N), 156.97 (2C, N-C(O)-N), 69.16, 69.83, 83.24 (10C, cyclopentadienyl rings); MS (m/z, %) : 384 (M+, 100%); Anal. Calcd. for C16H20N6O2Fe: C, 50.02; H, 5.25; N, 21.87. Found: C, 50.08; H, 5.21; N, 21.79.
General procedure for the preparation of hydrazones 6 and 8
A mixture of acetophenone (1, 1.00 equivalent) or 4-hydroxyacetophenone (2, 1.00 equivalent) and ethyl hydrazine carboxylate (1.20 equivalents) was dissolved in dry, hot chloroform (80 mL). When the reaction mixture started refluxing, two drops of concentrated hydrochloric acid were added and the mixture was then refluxed overnight with continuous removal of the water generated. The solvent was removed under vacuum and the residue was washed several times with diethyl ether or chloroform to remove excess reactants.
N’-[1-phenylethylidene]hydrazine carboxylic acid ethyl ester (6). White solid powder, yield = 83%; IR: ν 3192, 3051, 2975, 1732, 1540, 1035 cm-1; 1H-NMR (DMSO-d6): δ 1.24 (t, 3H, CH2CH3), 2.19 (s, 3H, CH3-C=N), 4.14 (q, 2H, CH2CH3), 7.27-7.33 (m, 3H, aromatic), 7.69-7.71 (m, 2H, aromatic), 10.09 (s, 1H, N-H); 13C-NMR (DMSO-d6): δ 13.93 (OCH2CH3), 14.71 (CH3-C=N), 60.61 (OCH2CH3), 148.93 (C=N), 154.34 (O-C(O)-N), 126.11, 128.32, 128.89 and 138.47 (aromatic carbons); MS (m/z, %): 206 (M+, 100%); Anal. Calcd. for C11H14N2O2: C, 64.06; H, 6.84; N, 13.58. Found: C, 63.70; H, 6.79; N, 13.60.
N’-[1-(hydroxyphenyl)ethylidene]hydrazine carboxylic acid ethyl ester (8). White solid powder, yield = quantitative; IR: ν 3385, 3263, 1726, 1610, 1501, 1406 cm-1; 1H-NMR (DMSO-d6): δ 1.28 (t, 3H, CH2CH3), 2.21 (s, 3H, CH3-C=N), 4.16 (q, 2H, CH2CH3), 7.28/7.70 (AA′BB′, 4H, aromatic), 9.97 (s, 1H, N-H); 13C-NMR (DMSO-d6): δ 13.97 (OCH2CH3), 14.77 (CH3-C=N), 60.58 (OCH2CH3), 148.94 (C=N), 154.47 (O-C(O)-N), 114.3/127.9 (CH, phenylene), 131.2/158.9 (Cq, phenylene); MS (m/z, %): 222 (M+, 100%); Anal. Calcd. for C11H14N2O3: C, 59.44; H, 6.34; N, 12.60. Found: C, 59.49; H, 6.40; N, 12.66.
N’-[1-(hydroxyphenyl)ethylidene]hydrazine tosylate (9).
Acetophenone (2.50 mmol) was added to a warm solution of p-toluenesulfonic acid hydrazide (2.70 mmol) in dry ethanol (30 mL). The solution was refluxed for 1.0 hr and then concentrated to one-half of its original volume. After cooling the reaction mixture to room temperature, the product precipitated as a colorless powder which was washed with a small amount of cooled ethanol and dried to afford the title compound in 86% yield; IR: ν 3385, 3263, 1726, 1610, 1501, 1406 cm-1; 1H-NMR (DMSO-d6): δ 2.21/2.38 (2s, 6H, CH3), 6.84/7.73 (AA′BB′, 4H, aromatic), 7.37/7.54 (AA′BB′, 8H, toluene), 10.31 (s, 1H, N-H); 13C-NMR (DMSO-d6): δ 14.19 (CH3), 20.72 (CH3, toluene), 114.17/127.63 (CH, phenylene), 130.51/159.80 (Cq, phenylene), 127.71/129.83 (CH, toluene), 136.58/143.29 (Cq, toluene), 152.64 (C=N); MS (m/z, %): 304 (M+, 100%); Anal. Calcd. for C15H16N2O3S: C, 59.19; H, 5.30; N, 9.24; S, 10.53. Found: C, 59.22; H, 5.26; N, 9.31; S, 10.59.
General procedure for the preparation of 1,2,3-selenadiazole derivatives 13, 15, 17, 19, 21
Each of the semicarbazones 7 (1.98 mmol), 10 (1.68 mmol), 11 (0.29 mmol) or 12 (1.00 mmol) or the hydrazones 6 or 8 (2.50 mmol), respectively, was dissolved in glacial acetic acid (40 mL) with vigorous stirring and gentle heating to 40-45 °C (in case of the hydrazone 6, the solution was stirred at room temperature). The solution was treated with selenium dioxide powder (2.70 mmol, 3.60 mmol, 0.57 mmol, 4.00 mmol or 2.72 mmol, respectively) and the mixture was kept under vigorous stirring. After ca. 2 min the color of the mixture becomes red. Monitoring of the reaction by TLC (eluent: 1:4 ethyl acetate-hexane) showed that the reaction was complete in 24 hr. Thes mixture were filtered and the filtrates poured into ice water and extracted with CDCl3 (3×50 mL). The combined organic layers were washed with saturated sodium hydrogen carbonate solution. dried over magnesium sulphate and the solvent was removed under vacuum to afford the crude title compounds, which were further purifed as indicated under each heading.
Ethyl-3,5-dihydro-4-phenyl-1-oxo-1,2,3-selenadiazole-2-carboxylate (13). The residue was washed with hexane and the insoluble solid was chromatographed using 1:3 ethyl acetate-hexane as eluent to give compound 13 as a pale yellow solid in 69% yield; IR: ν 3206, 2982, 1739, 1694, 1598, 1515, 1226, 726 cm-1; 1H-NMR (CDCl3): δ 1.29 (t, 3H, CH2CH3), 4.30 (q, 2H, CH2CH3), 7.20-7.22 (m, 3H, aromatic), 7.49-7.51 (m, 2H, aromatic), 8.62 (s, 1H, N-H), 9.71 (s, 1H, proton at C5 of selenadiazole ring); 13C-NMR (CDCl3): δ 14.45 (OCH2CH3), 63.19 (OCH2CH3), 126.29, 126.85, 128.35 and 129.78 for benzene carbons, 130.77 and 149.50 for C4 and C5 carbons of the 1,2,3-selenadiazole ring, 152.48 for O-C(O)-N; MS (m/z, %): 283 (M+, 100%); Anal. Calcd. For C11H12O3N2Se: C, 46.64; H, 4.24; N, 9.89. Found: C, 46.53; H, 4.12; N, 9.79.
4-Phenyl-[1,2,3]-selenadiazole (14). The residue was chromatographed using 1:4 ethyl acetate-hexane as mobile phase to give a yellow solid of compound 14 in 74% yield; IR: ν 3061, 1687, 1591, 1483, 1235 cm-1; 1H-NMR (CDCl3): δ 7.29-7.33 (m, 3H, aromatic), 7.54-7.56 (m, 2H, aromatic), 8.93 (s, 1H, selenadiazole ring); 13C-NMR (CDCl3): δ 126.41, 127.62, 127.90 and 130.73 for benzene carbons, 162.53 and 133.41 for C4 and C5 carbons of the selenadiazole ring); MS (m/z, %): 209 (M+, 100%); Anal. Calcd. For C8H6N2Se: C, 45.95; H, 2.89; N, 13.40. Found: C, 45.78; H, 2.93; N, 13.49.
4-[1,2,3]-Selenadiazole-4-yl-phenol (16). The residue was recrystalized from acetone/hexane to give a faint gray solid of compound 16 in 79% yield (when semicarbazone 7 is used) and 84% yield (when hydrazone 8 is used); IR: ν 3429, 3077, 1604, 1527, 1468, 1245, 803 cm-1; 1H-NMR (CDCl3): δ 2.10 (s, 1H, OH group), 6.90/7.90 (AA′BB′, 4H, phenylene), 9.20 (s, 1H, selenadiazole ring); 13C-NMR (CDCl3): δ 114.81/126.40 (CH, phenylene), 129.82/158.70 (Cq, phenylene), 162.41/133.29 (C4/C5, selenadiazole ring carbons); MS (m/z, %): 225 (M+, 100%); Anal. Calcd. For C8H6N2OSe: C, 42.69; H, 2.69; N, 12.44. Found: C, 42.74; H, 2.63; N, 12.48.
7,10,10-Trimethyl-3-selena-4,5-diaza-tricyclo[5.2.1.02,6]deca-2(6),4-diene (18). The crude product was chromatograhed using 1:4 ethyl acetate-hexane as mobile phase to give a pale yellow-brown solid of compound 18 in 50% yield; IR: ν 2949, 1540, 1420, 1232 cm-1; 1H-NMR (CDCl3): δ 0.72 (s, 3H at C9), 0.89 (s, 3H at C10), 1.05 (s, 3H at C8), 1.44/1.82 (2m, 4H at C5, C6), 2.29 (m, 1H at C4); 13C-NMR (CDCl3): δ (61.57, C1), (166.52, C2), (153.80, C3), (43.92, C4), (27.28, C5), (32.41, C6), (48.05, C7), (19.52, C8), (18.59, C9), (11.09, C10); MS (m/z, %): 238 (M+, 100%); Anal. Calcd. For C10H14N2Se: C, 49.80; H, 5.85; N, 11.61. Found C, 49.71; H, 5.84; N, 11.60.
[1,2,3]-Selenadiazole-4-yl-ferrocene (20). The crude product was purified as indicated for compound 18 to give an orange solid of compound 20 in 60% yield; IR: ν 3083, 1700, 1528, 1400, 1258, 816 cm‑1; 1H-NMR (CDCl3): δ 8.89 (s, 1H, proton at C5 selenadiazole ring), 4.17 (s, 5H, unsubstituted cyclopentadienyl ring), 4.47/5.00 (2d, 4H, monosubstituted cyclopentadienyl ring); 13C-NMR (CDCl3): δ 162.52 and 133.28 for C4 and C5 selenadiazole ring, 68.04, 69.45, 69.71 and 85.00 for the carbons of the mono and unsubstituted cyclopentadienyl rings; MS (m/z, %): 318 (M+, 100%); Anal. Calcd. For C12H10N2SeFe: C, 45.31; H, 3.49; N, 8.81. Found: C, 45.22; H, 3.47; N, 8.75.
Di-[1,2,3]-selenadiazole-4-yl-ferrocene (22). The crude product was chromatographed like compound 18 to give an orange solid of compound 22 in 64% yield; IR: ν 3075, 1693, 1528, 1403, 1248, 821 cm‑1; 1H-NMR (CDCl3): δ 8.91 (s, 2H, selenadiazole rings), 4.45/5.13 (2d, 8H, monosubstituted cyclopentadienyl rings); 13C-NMR (CDCl3): δ 162.68/133.32 (C4/C5, selenadiazole rings), 69.37, 69.82 and 85.26 for the carbons of the monosubstituted cyclopentadienyl rings; MS (m/z, %): 448 (M+, 100%); Anal. Calcd. For C14H10N4Se2Fe: C, 37.53; H, 2.25; N, 12.50. Found: C, 37.51; H, 2.29; N, 12.41.
General procedure for the preparation of 1,2,3-thiadiazole derivatives 15, 17, 19, 21 and 23 [13].
An excess amount of thionyl chloride was stirred at 0 °C and the hydrazones or semicarbazones 6, 7, 8, 10, 11 or 12 were added in several portions. The mixtures were stirred at room temperature overnight until no more hydrogen chloride was produced. The remaining thionyl chloride was evaporated under vacuum and the residue was washed with diethyl ether to give good yields of the corresponding 1,2,3-thiadiazoles as fine powders. A recrystallization from chloroform or dimethyl-sulfoxide was carried out when necessary.
4-Phenyl-[1,2,3]-thiadiazole (15). Beige powder, yield = 83%; IR: ν 3094, 1605, 1456, 1408, 1281, 1068, 921 cm-1; 1H-NMR (DMSO-d6): δ 7.28-7.31 (m, 3H, aromatic), 7.53-7.55 (m, 2H, aromatic), 8.89 (s, 1H, selenadiazole ring); 13C-NMR (DMSO-d6): δ 126.81, 127.42, 127.90 and 130.81 for benzene carbons, 161.31 and 134.60 for C4 and C5 carbons of the thiadiazole ring; MS (m/z, %): 162 (M+, 100%); Anal. Calcd. For C8H6N2S: C, 59.24; H, 3.73; N, 17.27, S, 19.77. Found: C, 45.78; H, 2.93; N, 13.49, S, 19.59.
4-[1,2,3]-Thiadiazole-4-yl-phenol (17). Pale brown powder, yield = 76%; IR: ν 3413, 3109, 1597, 1501, 1450, 1411, 1278, 1073, 927 cm-1; 1H-NMR (DMSO-d6): δ 2.16 (s, 1H, OH group), 7.21/7.56 (AA′BB′, 4H, phenylene), 8.83 (s, 1H, thiadiazole ring); 13C-NMR (DMSO-d6): δ 116.21/127.80 (CH, phenylene), 130.61/159.11 (Cq, phenylene), 161.70/133.80 (C4/C5, thiadiazole ring carbons); MS (m/z, %): 178 (M+, 100%); Anal. Calcd. For C8H6N2OS: C, 53.92; H, 3.39; N, 15.72, S, 17.99. Found: C, 53.71; H, 3.28; N, 15.61, S, 17.93.
7,10,10-Trimethyl-3-thia-4,5-diaza-tricyclo[5.2.1.02,6]deca-2(6),4-diene (19). Pale yellow powder, yield = 58%; IR: ν 2963, 1609, 1459, 1406, 1283, 925 cm-1; 1H-NMR (CDCl3): δ 0.76 (s, 3H at C9), 0.93 (s, 3H at C10), 1.08 (s, 3H at C8), the peaks for the other protons were observed between (1.43-2.38); 13C-NMR (CDCl3): δ 61.64 (C1), 166.81 (C2), 154.04 (C3), 44.11 (C4), 27.26 (C5), 32.40 (C6), 48.08 (C7), 19.73 (C8), 18.91 (C9), 11.04 (C10); MS (m/z, %): 194 (M+, 100%); Anal. Calcd. For C10H14N2S: C, 61.82; H, 7.26; N, 14.42. Found C, 61.85; H, 7.28; N, 14.46.
[1,2,3]-Thiadiazole-4-yl-ferrocene (21). Yellow powder, yield = 66%; IR: ν 3068, 1608, 1458, 1413, 1274, 927 cm-1; 1H-NMR (CDCl3): δ 8.67 (s, 1H, proton at C5 thiadiazole ring), 4.15 (s, 5H, unsubstituted cyclopentadienyl ring), 4.43/5.10 (2d, 4H, monosubstituted cyclopentadienyl ring); 13C-NMR (CDCl3): δ 162.36 and 133.49 for C4 and C5 thiadiazole ring, 68.12, 69.53, 69.68 and 84.89 for the carbons of the mono- and unsubstituted cyclopentadienyl rings; MS (m/z, %): 270 (M+, 100%); Anal. Calcd. For C12H10N2SFe: C, 53.36; H, 3.73; N, 10.37. Found: C, 53.61; H, 3.70; N, 10.42.
Di-[1,2,3]-thiadiazole-4-yl-ferrocene (23). Yellow powder, yield = 61%; IR: ν 3081, 1613, 1457, 1420, 1268, 926 cm-1; 1H-NMR (CDCl3): δ 8.72 (s, 2H, thiadiazole rings), 4.47/5.11 (2d, 8H, monosubstituted cyclopentadienyl rings); 13C-NMR (CDCl3): δ 161.97/133.14 (C4/C5, selenadiazole rings), 69.48, 69.71 and 84.99 for the carbons of the monosubstituted cyclopentadienyl rings; MS (m/z, %): 354 (M+, 100%); Anal. Calcd. For C14H10N4S2Fe: C, 47.47; H, 2.84; N, 15.82. Found: C, 47.56; H, 2.90; N, 15.84.