Unexpected Syntheses of seco-Cyclopropyltetrahydroquinolines >From a Radical 5-Exo-Trig Cyclization Reaction: Analogs of CC-1065 and the Duocarmycins
Abstract
:Introduction
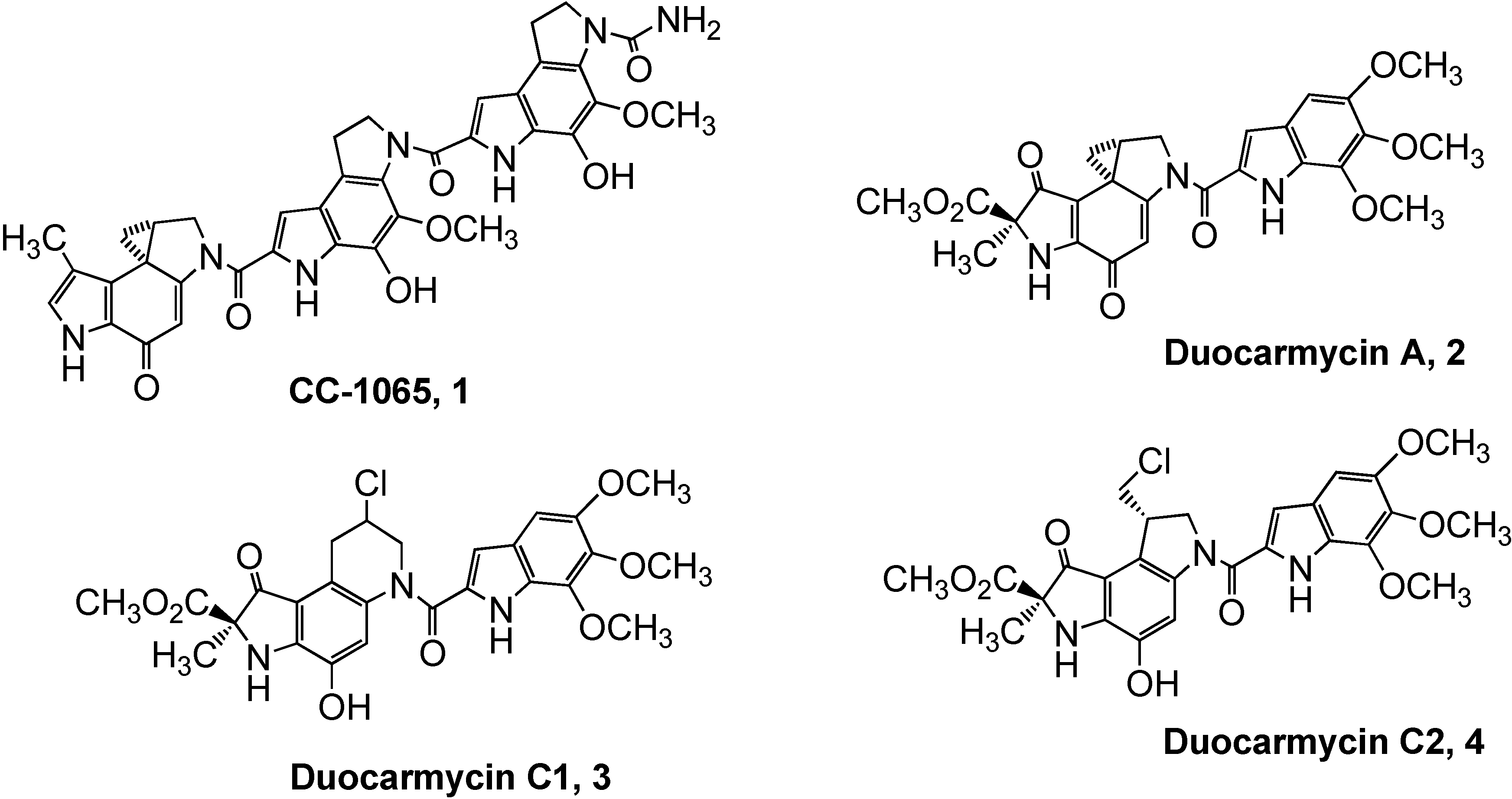
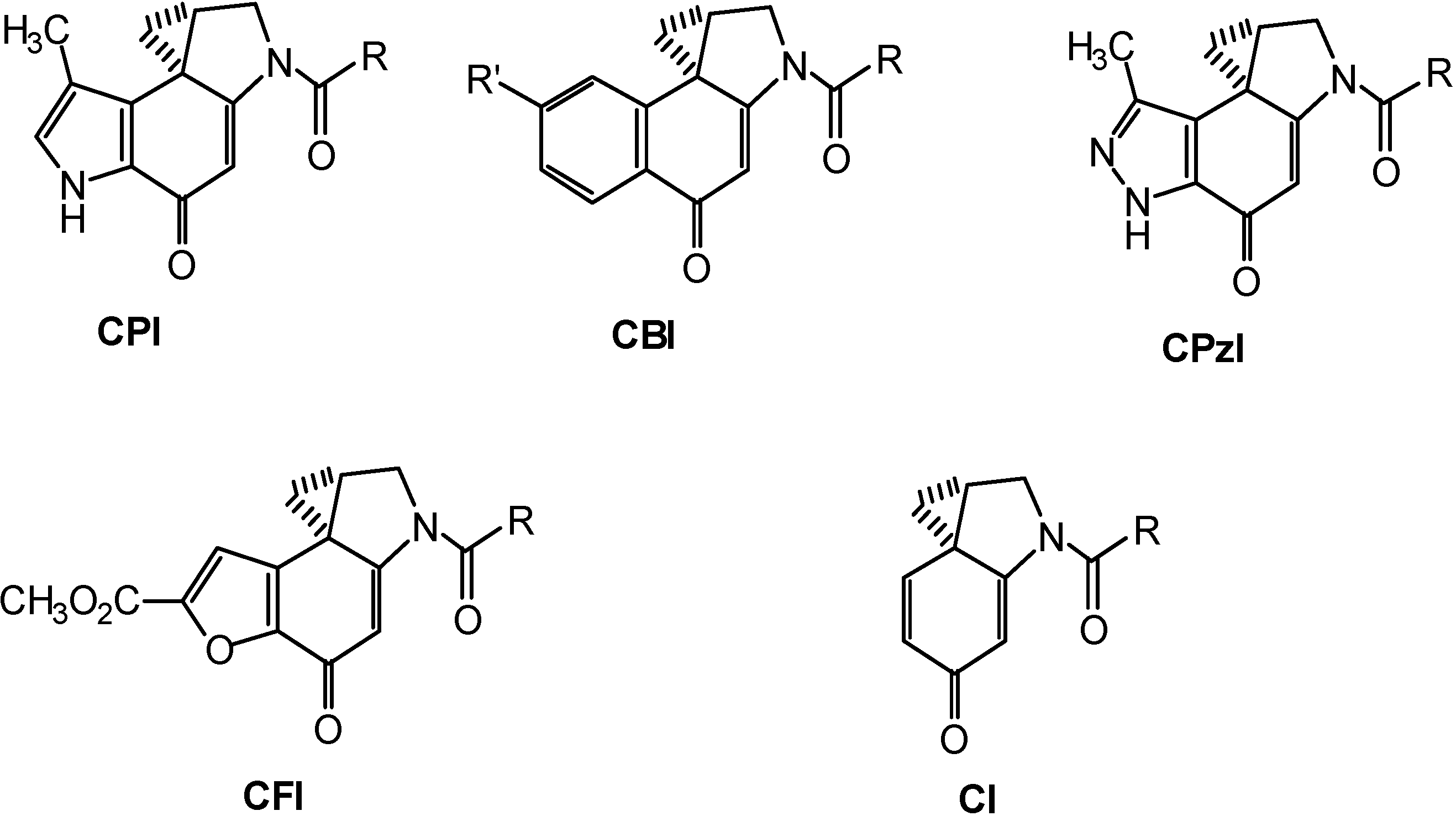
Results and Discussion
| Reactant | Indoline (6) | Quinoline (7) | Ratio of 6:7 |
|---|---|---|---|
| 5a | 40% | 48% | 1:1.2 |
| 5b | 37 | 32 | 1.2:1 |
| 5c | 35 | 32 | 1.1:1 |
| 5d | 29 | 19 | 1.5:1 |
| 5e | 86 | none isolated | not calculated |

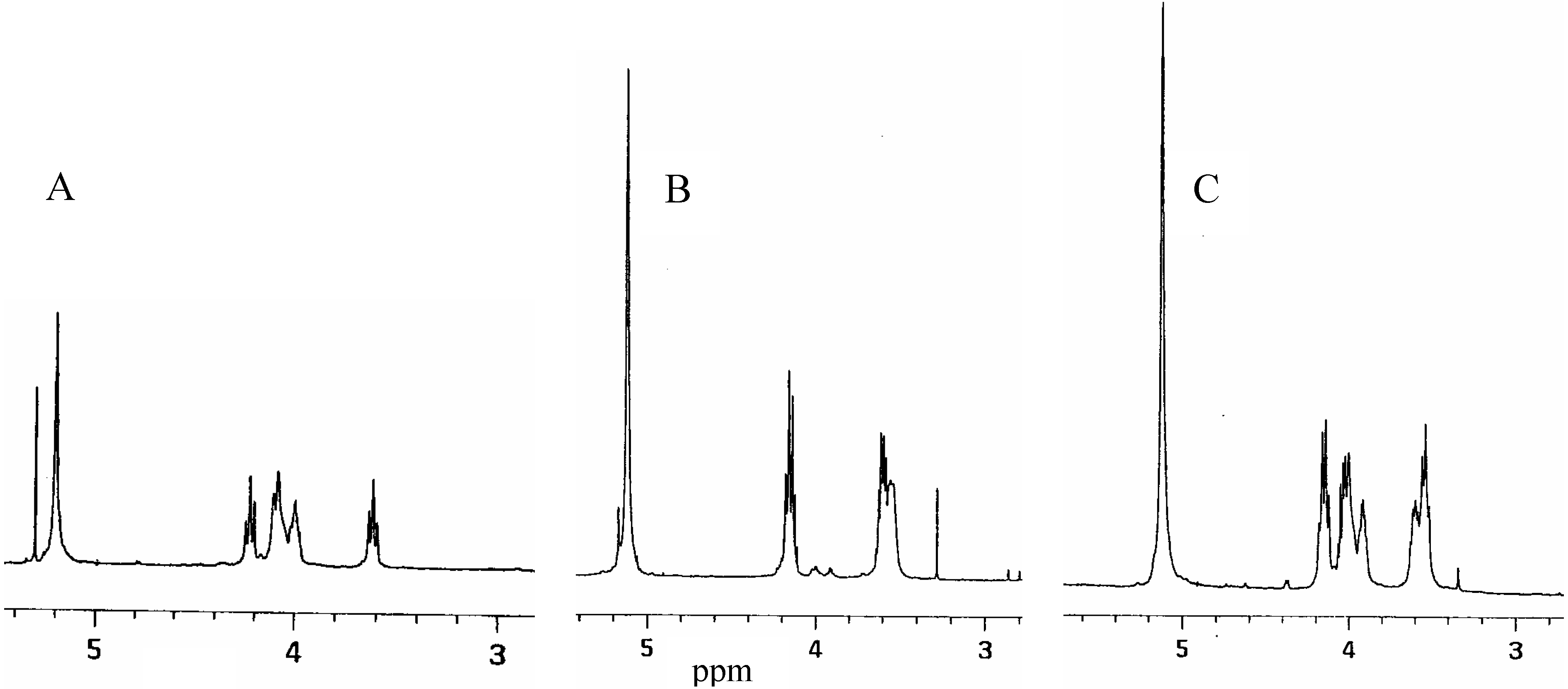
Acknowledgements
Experimental
General
General Procedure for the Free Radical 5-Exo-Trig Cyclization
References
- Li, L.H.; Wallace, T.L.; DeKoning, T.F.; Warpehoski, M.A.; Kelly, R.C.; Prairie, M.D.; Drueger, W.C. Invest. New Drugs 1987, 5, 329.Li, L.H.; Kelly, R.C.; Warpehoski, M.A.; McGovern, J.P.; Gebhard, I.; DeKoning, T.F. Invest. New Drugs 1991, 9, 137.(c)Warpehoski, M. Advances in DNA Sequence Specific Agents; Hurley, L.H., Ed.; JAI Press, Inc.: Greenwich, CT, 1992; Vol. 1, pp. 217–245. [Google Scholar]
- Boger, D.L.; Garbaccio, R.M. Acc. Chem. Res. 1999, 32, 1043.Boger, D.L.; Johnson, D.S. Angew. Chem. Int. Ed. Engl. 1996, 35, 1438.Boger, D.L.; Johnson, D.S. Proc. Natl. Acad. Sci. USA 1995, 92, 3642.Boger, D.L. Acc. Chem. Res. 1995, 28, 20.Boger, D.L. Chemtracts: Org. Chem. 1991, 4, 329.
- Boger, D.L.; Boyce, C.W.; Johnson, D.S. Bioorg. Med. Chem. Lett. 1997, 7, 233. [CrossRef]
- Boger, D.L.; Johnson, D.S.; Wassidlo, W. Bioorg. Med. Chem. Lett. 1994, 4, 631.Wang, Y.; Yuan, H.; Ye, W.; Wright, S.C.; Wang, H.; Larrick, J.W. J. Med. Chem. 2000, 43, 1541.
- Hightower, R.D.; Sevin, B.U.; Pevras, J.P.; Untch, M.; Angioli, R.; Averette, H. Gynecol. Oncol. 1992, 42, 186.Cote, S.; Momparler, R.L. Anti-Cancer Drugs 1993, 4, 327.Fleming, G.F.; Ratain, S.M.; O’Brien, R.L.; Schilsky, P.C.; Ramos, R.; Richards, J.M.; Vogelzang, N.J.; Kasunic, D.A.; Earhart, R.H. J. Natl. Cancer Inst. 1994, 86, 368.Foster, B.J.; Larusso, P.M.; Poplin, E.; Zalupski, M.; Valdivieso, M.; Wozniak, A.; Flaherty, L.; Kasunic, D.A.; Earhart, R.H.; Baker, L.H. Invest. New Drugs 1996, 13, 321.Burris, H.A.; Dieras, V.C.; Tunca, M.; Earhart, R.H.; Earhart, J.R.; Rodriguez, G.L.; Shaffer, D.S.; Fields, S.M.; Campbell, E.; Schaaf, L.; Kasunic, D.; Von Hoff, D.D. Anti-Cancer Drugs 1997, 8, 588.
- Li, L.H.; Dekoning, J.F.; Kelly, R.C.; Krueger, W.C.; McGovern, J.P.; Padbury, G.E.; Petzold, G.L.; Wallace, T.L.; Ouding, R.J.; Praire, M.D.; Gebhard, I. Cancer Res. 1992, 52, 4904.van Telligen, O.; Punt, C.J.A.; Awada, A.; Wagener, D.J.T.; Piccart, M.J.; Groot, Y.; Schaaf, L.J.; Henrar, R.E.C.; Nooijen, W.J.; Beijnen, J.H. Cancer Chemother. Pharmacol. 1998, 41, 366.
- Nagamura, S.; Kobayashi, E.; Gomi, K.; Saito, H. Bioorg. Med. Chem. Lett. 1996, 6, 2147, and references therein.Alberts, S.R.; Erlichman, C.; Reid, J.M.; Sloan, J.A.; Ames, M.M.; Richardson, R.L.; Goldberg, R.M. Clin. Cancer Res. 1998, 4, 2111.
- Walker, D.L.; Reid, F.M.; Ames, M.M. Cancer Chemother. Pharmacol. 1994, 34, 317–322.Volpe, D.A.; Tomaszewski, J.E.; Parchment, R.E.; Garg, A.; Flora, K.P.; Murphy, M.J.; Grieshaber, C.K. Cancer. Chemother. Pharmacol. 1996, 39, 143.Pitot IV, H.C.; Erlichman, C.; Reid, J.M.; Sloan, J.A.; Ames, M.M.; Bagniewski, P.G.; Atherton-Skaaf, P.; Adjei, A.A.; Rubi, J.; Rayson, D.; Goldberg, R.M. Proc. Am. Assoc. Cancer Res. 1999, 40, 91.
- Boger, D.L.; McKie, J.A. J. Org. Chem. 1995, 60, 1271.Boger, D.L.; Yun, W.; Han, N. Bioorg. Med. Chem. 1995, 3, 1429.
- Baraldi, P.G.; Cacciari, B.; Pinedo de las Infantas, M.J.; Romagnoli, R.; Spatullo, G.; Cozzi, P.; Mongelli, N. Anti-Cancer Drug Design 1997, 12, 67.
- Mohamadi, F.; Spees, M.M.; Staten, G.S.; Marder, P.; Kipka, J.K.; Johnson, D.A.; Boger, D.L.; Zarrinmayeh, H. J. Med. Chem. 1994, 37, 232.Patel, V.F.; Andis, S.L.; Enkema, J.K.; Johnson, D.A.; Kennedy, J.H.; Mohamadi, F.; Schultz, R.M.; Soose, D.J.; Spees, M.M. J. Org. Chem. 1997, 62, 8868.
- Boger, D.L.; Munk, S.A.; Zarrinmayeh, H.; Ishizaki, T.; Haught, J.; Bina, M. Tetrahedron 1991, 47, 2661.Boger, D.L.; Ishizaki, T.; Zarrinmayeh, H.; Munk, S.A.; Kitos, P.A.; Suntorwart, O. J. Am. Chem. Soc. 1990, 112, 8961.
- Howard, T.T.; Lingerfelt, B.M.; Purnell, B.L.; Scott, A.E.; Price, C.A.; Townes, H.M.; McNulty, L.; Handl, H.L.; Summerville, K.; Hudson, S.J.; Bowen, P.J.; Kiakos, K.; Hartley, J.A.; Lee, M. Bioorg. Med. Chem. 2002, 10, 2941. [CrossRef]
- Boger, D.L.; Boyce, C.W.; Garbaccio, R.M.; Searcey, M. Tetrahedron Lett. 1998, 39, 2227.
- Sample Availability: Available from authors
© 2004 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Pati, H.; Forrest, L.; Townes, H.; Lingerfelt, B.; McNulty, L.; Lee, M. Unexpected Syntheses of seco-Cyclopropyltetrahydroquinolines >From a Radical 5-Exo-Trig Cyclization Reaction: Analogs of CC-1065 and the Duocarmycins. Molecules 2004, 9, 125-133. https://doi.org/10.3390/90300125
Pati H, Forrest L, Townes H, Lingerfelt B, McNulty L, Lee M. Unexpected Syntheses of seco-Cyclopropyltetrahydroquinolines >From a Radical 5-Exo-Trig Cyclization Reaction: Analogs of CC-1065 and the Duocarmycins. Molecules. 2004; 9(3):125-133. https://doi.org/10.3390/90300125
Chicago/Turabian StylePati, Hari, Lori Forrest, Heather Townes, Brian Lingerfelt, LuAnne McNulty, and Moses Lee. 2004. "Unexpected Syntheses of seco-Cyclopropyltetrahydroquinolines >From a Radical 5-Exo-Trig Cyclization Reaction: Analogs of CC-1065 and the Duocarmycins" Molecules 9, no. 3: 125-133. https://doi.org/10.3390/90300125




