The Effect of Treadmill Training Pre-Exercise on Glutamate Receptor Expression in Rats after Cerebral Ischemia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physiological Variables
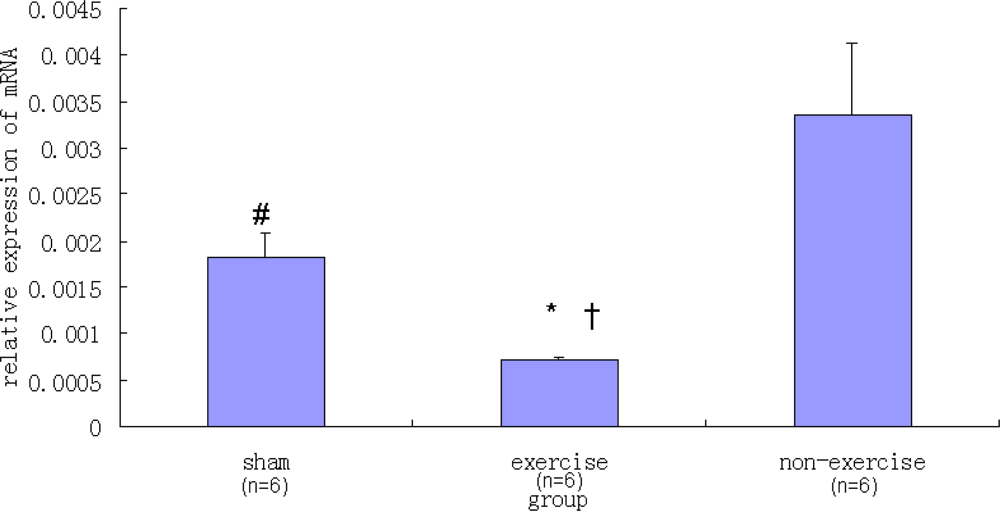
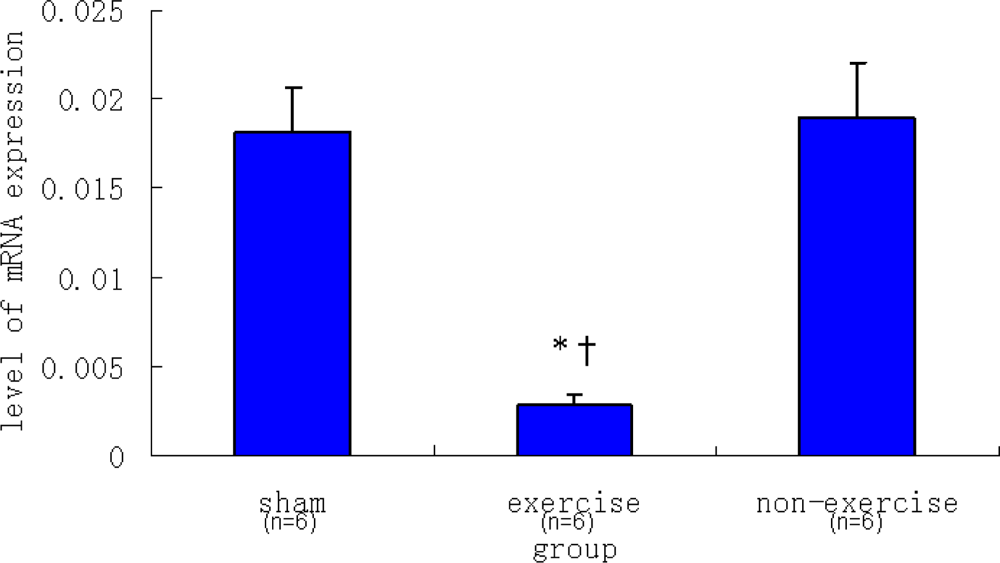
2.2. Relative NR2B and mGluR5 mRNA Expression Levels among the Three Groups
2.3. Behavioral Scores
2.4. Infarct Volume
2.5. Discussion
3. Experimental Methods
3.1. Animals
3.2. Treadmill Training
3.3. Rat MCAO Model
3.4. Evaluation of Behavioral Score
3.5. Determination of Brain Infarction Volume
3.6. Tissue Processing
3.7. Reverse Transcription
3.8. Quantitative Real-Time PCR
3.9. Statistical Analysis
4. Conclusions
Abbreviations:
| mGluR5 | metabotropic glutamate receptor 5 |
| AMPA | α-amino-3-hydroxi-5-methyl-4-isoxazol pro-pionic acid |
| NMDA | N-methyl-d-aspartate; |
| NR2B | NMDA receptor subunit type 2B |
| MPEP | 2-[11C]Methyl-6-(2-phenylethynyl)pyridine |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
Acknowledgments
References
- Moseley, AM; Stark, A; Cameron, ID; Pollock, A. Treadmill training and body weight support for walking after stroke. Stroke 2003, 34, 3006, doi:10.1161/01.STR.0000102415.43108.66,. [Google Scholar]
- Endres, M; Gertz, K; Lindauer, U; Katchanov, J; Schultze, J; Schrock, H; Nickenig, G; Kuschinsky, W; Dirnagl, U; Laufs, U. Mechanisms of stroke protection by physical activity. Ann. Neurol 2003, 54, 582–590. [Google Scholar]
- Li, J; Luan, XD; Clark, JC; Rafols, JA; Ding, YC. Neuroprotection against transient cerebral ischemia by exercise pre-conditioning in rats. Neurol. Res 2004, 26, 404–408. [Google Scholar]
- Wang, RY; Yang, YR; Yu, SM. Protective effects of treadmill training on infarction in rats. Brain Res 2001, 922, 140–143. [Google Scholar]
- Ding, YH; Young, CN; Luan, XD; Li, J; Rafols, JA; Clark, JC; McAllister, JP; Ding, YC. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta. Neuropathol 2005, 109, 237–246. [Google Scholar]
- Kitagawa, K; Matsumoto, M; Tagaya, M; Hata, R; Ueda, H; Niinobe, M; Handa, N; Fukunaga, R; Kimura, K; Mikoshiba, K; Kamada, T. Ischemic tolerance phenomenon found in the brain. Brain Res 1990, 528, 21–24. [Google Scholar]
- Gidday, JM; Fitzgibbons, JC; Shah, AR; Park, TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci. Lett 1994, 168, 221–224. [Google Scholar]
- PerezPinzon, MA; Mumford, PL; Rosenthal, M; Sick, TJ. Anoxic preconditioning in hippocampal slices: Role of adenosine. Neuroscience 1996, 75, 687–694. [Google Scholar]
- Guo, M; Lin, V; Davis, W; Huang, T; Carranza, A; Sprague, S; Reyes, R; Jimenez, D; Ding, YC. Preischemic induction of TNF-alpha by physical exercise reduces blood-brain barrier dysfunction in stroke. J. Cereb. Blood. Flow. Metab 2008, 28, 1422–1430. [Google Scholar]
- Guyot, LL; Diaz, FG; O'Regan, MH; McLeod, S; Park, H; Phillis, JW. Real-time measurement of glutamate release from the ischemic penumbra of the rat cerebral cortex using a focal middle cerebral artery occlusion model. Neurosci. Lett 2001, 299, 37–40. [Google Scholar]
- Matsumoto, K; Graf, R; Rosner, G; Taguchi, J; Heiss, WD. Elevation of neuroactive substances in the cortex of cats during prolonged focal ischemia. J. Cereb. Blood. Flow. Metab 1993, 13, 586–594. [Google Scholar]
- Chen, M; Lu, TJ; Chen, XJ; Zhou, Y; Chen, Q; Feng, XY; Xu, L; Duan, WH; Xiong, ZQ. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 2008, 39, 3042–3048. [Google Scholar]
- Szydlowska, K; Kaminska, B; Baude, A; Parsons, CG; Danysz, W. Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo. Eur. J. Pharmacol 2007, 554, 18–29. [Google Scholar]
- Jia, J; Hu, YS; Wu, Y; Liu, G; Yu, HX; Zheng, QP; Zhu, DN; Xia, CM; Cao, ZJ. Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia. Life Sci 2009, 84, 505–511. [Google Scholar]
- Jia, J; Hu, YS; Wu, Y; Liu, G; Yu, HX; Zhu, DN; Xia, CM; Cao, ZJ; Zhang, X; Guo, QC. Treadmill pre-training suppresses the release of glutamate resulting from cerebral ischemia in rats. Exp. Brain. Res 2010, 204, 173–179. [Google Scholar]
- Ozawa, S; Kamiya, H; Tsuzuki, K. Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol 1998, 54, 581–618. [Google Scholar]
- Spooren, W; Ballard, T; Gasparini, F; Amalric, M; Mutel, V; Schreiber, R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: Behavioural characterization and implications for the treatment of CNS disorders. Behav. Pharmacol 2003, 14, 257–277. [Google Scholar]
- Mannaioni, G; Marino, MJ; Valenti, O; Traynelis, SF; Connm, PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci 2001, 21, 5925–5934. [Google Scholar]
- Ireland, DR; Abraham, WC. Group I mGluRs Increase Excitability of Hippocampal CA1 Pyramidal Neurons by a PLC-Independent Mechanism. J. Neurophysiol 2002, 88, 107–116. [Google Scholar]
- Copani, A; Bruno, V; Battaglia, G; Leanza, G; Pellitteri, R; Russo, A; Stanzani, S; Nicoletti, F. Activation of metabotropic glutamate receptors protects cultured neurons against apoptosis induced by beta-amyloid peptide. Mol. Pharmacol 1995, 47, 890–897. [Google Scholar]
- Nicoletti, F; Bruno, V; Catania, MV; Battaglia, G; Copani, A; Barbagallo, G; Cena, V; Sanchez-Prieto, J; Spano, PF; Pizzi, M. Group-I metabotropic glutamate receptors: Hypotheses to explain their dual role in neurotoxicity and neuroprotection. Neuropharmacology 1999, 38, 1477–1484. [Google Scholar]
- Allen, JW; Knoblach, SM; Faden, AI. Activation of group I metabotropic glutamate receptors reduces neuronal apoptosis but increases necrotic cell death in vitro. Cell Death Differ 2000, 7, 470–476. [Google Scholar]
- Bao, WL; Williams, AJ; Faden, AI; Tortella, FC. Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia. Brain Res 2001, 922, 173–179. [Google Scholar]
- Movsesyan, VA; O’Leary, DM; Fan, L; Bao, W; Mullins, PG; Knoblach, SM; Faden, AI. mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther 2001, 296, 41–47. [Google Scholar]
- Kinga, S; Bozena, K; Andrea, B; Chris, GP; Wojciech, D. Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo. Eur. J. Pharmacol 2007, 554, 18–29. [Google Scholar]
- Barone, FC; White, RF; Spera, PA; Ellison, J; Currie, RW; Wang, X; Feuerstein, GZ. Ischemic preconditioning and brain tolerance: Temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke 1998, 29, 1937–1950. [Google Scholar]
- Miao, B; Yin, XH; Pei, DS; Zhang, QG; Zhang, GY. Neuroprotective effects of preconditioning ischemia on ischemic brain injury through down-regulating activation of JNK1/2 via N-methyl-D-aspartate receptor-mediated AKT1 activation. J. Biol. Chem 2005, 280, 21693–21699. [Google Scholar]
- Wang, RM; Yang, F; Zhang, YX. Preconditioning-induced activation of ERK5 is dependent on moderate Ca2+ influx via NMDA receptors and contributes to ischemic tolerance in the hippocampal CA1 region of rats. Life Sci 2006, 79, 1839–1846. [Google Scholar]
- Bond, A; Lodge, D; Hicks, CA; Ward, MA; O’Neill, MJ. NMDA receptor antagonism, but not AMPA receptor antagonism attenuates induced ischaemic tolerance in the gerbil hippocampus. Eur. J. Pharmacol 1999, 380, 91–99. [Google Scholar]
- Grabb, MC; Choi, DW. Ischemic tolerance in murine cortical cell culture: Critical role for NMDA receptors. J. Neurosci 1999, 19, 1657–1662. [Google Scholar]
- Birmingham, K. Future of neuroprotective drugs in doubt. Nat. Med 2002, 8, 5. [Google Scholar]
- Ikonomidou, C; Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet. Neurol 2002, 1, 383–386. [Google Scholar]
- Zea, LE; Weinstein, PR; Carlson, S. Reversible middle cerebral artery occlusion without craniectomy in rat. Stroke 1989, 20, 84–91. [Google Scholar]
- Ding, YH; Ding, YC; Li, J; Bessert, DA; Rafols, JA. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol. Res 2006, 28, 184–189. [Google Scholar]
- Christoffersen, NR; Wrang, ML; Alsbo, CW; Diemer, NH. mGluR2 AND mGluR5 mRNA in ischemia tolerant rat brain. Soc Neurosci 2002. Abstract No. 202.214.. [Google Scholar]
- Dos-Anjos, S; Martinez-Villayandre, B; Montori, S; Perez-Garcia, CC; Fernandez-Lopez, A. Early modifications in N-methyl-D-aspartate receptor subunit mRNA levels in an oxygen and glucose deprivation model using rat hippocampal brain slices. Neuroscience 2009, 164, 1119–1126. [Google Scholar]
- Livak, KJ; Schmittgen, TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–DDCt method. Methods 2001, 25, 402–408. [Google Scholar]


| Before ischemia | After ischemia | |
|---|---|---|
| Sham | ||
| pH | 7.22 ± 0.01 | 7.23 ± 0.01 |
| pCO2 (mmHg) | 42.8 ± 3.5 | 43.1 ± 1.9 |
| pO2 (mmHg) | 103.8 ± 5.64 | 103.6 ± 2.5 |
| Exercise | ||
| pH | 7.22 ± 0.02 | 7.22 ± 0.03 |
| pCO2 (mmHg) | 42.1 ± 2.2 | 42.5 ± 4.5 |
| pO2 (mmHg) | 102.9 ± 3.5 | 104.0 ± 3.3 |
| Non-exercise | ||
| pH | 7.23 ± 0.04 | 7.22 ± 0.05 |
| pCO2 (mmHg) | 41.9 ± 3.1 | 42.6 ± 2.4 |
| pO2 (mmHg) | 102.8 ± 3.8 | 103.1 ± 5.7 |
| Gene Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|
| NR2B: TCCGTCTTTCTTATGTGGATATGC CCTCTAGGCGGACAGATTAAGG | |
| mGluR: 5 VCCCTAAGCTCCAACGGAAAAT VTGATGACCGCCGTTTGGT | |
| GAPDH: GGGCAGCCCAGAACATCA TGTCCGTATGGCTTCATTGATG | |
| Group | NR2B | mGluR5 |
|---|---|---|
| Sham | 0.00182 ± 0.00026 | 0.01816 ± 0.00241 |
| Exercise | 0.00070 ± 0.00004 | 0.00286 ± 0.00061 |
| Non-exercise | 0.00335 ± 0.00076 | 0.01896 ± 0.00312 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, F.; Jia, J.; Wu, Y.; Hu, Y.; Wang, Y. The Effect of Treadmill Training Pre-Exercise on Glutamate Receptor Expression in Rats after Cerebral Ischemia. Int. J. Mol. Sci. 2010, 11, 2658-2669. https://doi.org/10.3390/ijms11072658
Zhang F, Jia J, Wu Y, Hu Y, Wang Y. The Effect of Treadmill Training Pre-Exercise on Glutamate Receptor Expression in Rats after Cerebral Ischemia. International Journal of Molecular Sciences. 2010; 11(7):2658-2669. https://doi.org/10.3390/ijms11072658
Chicago/Turabian StyleZhang, Feng, Jie Jia, Yi Wu, Yongshan Hu, and Yang Wang. 2010. "The Effect of Treadmill Training Pre-Exercise on Glutamate Receptor Expression in Rats after Cerebral Ischemia" International Journal of Molecular Sciences 11, no. 7: 2658-2669. https://doi.org/10.3390/ijms11072658





